Cholinesterase inhibitors for the treatment of Alzheimer's disease:: getting on and staying on
- PMID: 24944370
- PMCID: PMC4052996
- DOI: 10.1016/S0011-393X(03)00059-6
Cholinesterase inhibitors for the treatment of Alzheimer's disease:: getting on and staying on
Abstract
Background: Cholinesterase (ChE) inhibitors currently used in the treatment of Alzheimer's disease (AD) are the acetylcholinesterase (AChE)-selective inhibitors, donepezil and galantamine, and the dual AChE and butyrylcholinesterase (BuChE) inhibitor, rivastigmine. In addition to differences in selectivity for AChE and BuChE, ChE inhibitors also differ in pharmacokinetic and pharmacodynamic properties, and these differences could significantly impact on safety, tolerability, and efficacy.
Objective: The aim of this article was to provide an overview of the ChE inhibitors widely used in AD, focusing on key pharmacologic differences among agents and how these may translate into important differences in safety, tolerability, and efficacy in clinical practice.
Methods: Using published literature collected over time by the author, a review was conducted, focusing on the pharmacology and clinical data of donepezil, galantamine, and rivastigmine.
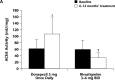
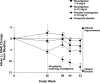
Results: All ChE inhibitors have the potential to induce centrally mediated cholinergic adverse events (AEs), such as nausea and vomiting, if the dose is increased too rapidly or in increments that are too large. These AEs, which are most likely to occur during the "getting on," or dose-escalation, phase of treatment, may result in patients discontinuing treatment early without achieving optimum therapeutic benefit. To reduce the incidence of these AEs, a slow dose-escalation schedule has been established in clinical practice, consisting of a "start low, go slow" procedure with a minimum of 4 weeks between dose increases. After "getting on" treatment, maintaining treatment in the long term, or "staying on," may be achieved with good safety, tolerability, and sustained symptomatic efficacy across the key symptom domains (activities of daily living, behavior, and cognition).
Conclusions: ChE inhibitors provide symptomatic benefit in AD across key symptom domains. Factors influencing the safety, tolerability, and efficacy of these agents in clinical practice include ChE enzymes inhibited, brain and brain-region ChE selectivity, and metabolism route. Class-specific cholinergic AEs can be minimized using slow, flexible dose escalation.
Keywords: Alzheimer's disease; acetylcholinesterase; butyrylcholinesterase; cholinesterase inhibitors; safety and tolerability.
Figures


References
-
- Bartus R.T, Dean R.L, III, Beer B, Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. - PubMed
-
- Foster R.H, Plosker G.L. Donepezil. Pharmacoeconomic implications of therapy. Pharmacoeconomics. 1999;16:99–114. - PubMed
-
- Lamb H.M, Goa K.L. Rivastigmine: A pharmacoeconomic review of its use in Alzheimer's disease. Pharmacoeconomics. 2001;19:303–318. - PubMed
-
- Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol. 1998;1:55–65.
LinkOut - more resources
Full Text Sources

