Efficacy and tolerability of delapril plus indapamide versus lisinopril plus hydrochlorothiazide combination treatments in mild to moderate hypertension: a multicenter, randomized clinical study
- PMID: 24944377
- PMCID: PMC4053002
- DOI: 10.1016/S0011-393X(03)00084-5
Efficacy and tolerability of delapril plus indapamide versus lisinopril plus hydrochlorothiazide combination treatments in mild to moderate hypertension: a multicenter, randomized clinical study
Abstract
Background: Several studies have shown that antihypertensive monotherapy is commonly insufficient to control blood pressure (BP) in hypertensive patients and that concomitant use of ≥2 drugs is necessary in ∼50% of these patients. The combination of an angiotensin-converting enzyme (ACE) inhibitor and a diuretic, delapril plus indapamide (D + I), has been shown to be effective and tolerable, with no interaction between the 2 components. Another widely used combination of ACE inhibitor and diuretic is lisinopril plus hydrochlorothiazide (L + H).
Objectives: The aims of this study were to confirm the antihypertensive efficacy and tolerability of the fixed combination of D + I in mild to moderate hypertension, and to compare its therapeutic efficacy and tolerability with that of L + H.
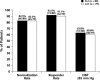
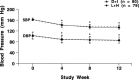
Methods: The antihypertensive efficacy and tolerability of a fixed combination of D + I (30-mg + 2.5-mg tablets once daily) or L + H (20-mg + 12.5-mg tablets once daily) in patients with mild to moderate hypertension were compared in a multinational, multicenter, randomized, 2-armed, parallel-group study. Eligible patients were aged 18 to 75 years and had a diastolic blood pressure (DBP) 95 to 115 mm Hg and a systolic blood pressure (SBP) ≤180 mm Hg, both measured in the sitting position. After a single-blind, placebo run-in period of 2 weeks, patients were randomized to receive 1 of the 2 treatments for a 12-week period. The primary efficacy end point was the BP normalization rate (ie, the percentage of patients with a sitting DBP ≤90 mm Hg) after 12 weeks of treatment. Secondary end points were as follows: (1) the responder rate (ie, the percentage of patients whose sitting DBP was reduced by ≥10 mm Hg from baseline or had a DBP ≤90 mm Hg after 12 weeks of treatment), (2) the percentage of patients with a DBP ≤85 mm Hg, and (3) changes in sitting SBP and DBP after 4, 8, and 12 weeks of treatment.
Results: A total of 159 hypertensive patients (88 women, 71 men) were randomized to receive D + I (44 women, 36 men; mean [SD] age, 53 [(11)] years) or L + H (44 women, 35 men; mean [SD] age, 55 [(10)] years). No significant between-group differences were found in any of the primary or secondary end points of the study. Both combinations induced a significant reduction in sitting DBP and SBP from baseline (P<0.001 for both groups at week 12), without significant differences between the groups. Five mild to moderate adverse drug reactions (ADRs) occurred in each treatment group. No patient dropped out of the study because of an ADR.
Conclusion: This study showed no difference between D + I and L + H interms of antihypertensive efficacy or tolerability in patients with mild to moderate hypertension.
Keywords: combination therapy; delapril; hydrochlorothiazide; hypertension; indapamide; lisinopril.
Figures
Similar articles
-
Delapril plus indapamide: a review of the combination in the treatment of hypertension.Clin Drug Investig. 2007;27(6):367-80. doi: 10.2165/00044011-200727060-00001. Clin Drug Investig. 2007. PMID: 17506588 Review.
-
Efficacy and safety of delapril plus manidipine compared with enalapril plus hydrochlorothiazide in mild to moderate essential hypertension: results of a randomized trial.Clin Ther. 2004 Sep;26(9):1419-26. doi: 10.1016/j.clinthera.2004.09.018. Clin Ther. 2004. PMID: 15531004 Clinical Trial.
-
Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: the VAST study.Clin Ther. 2005 May;27(5):578-87. doi: 10.1016/j.clinthera.2005.05.006. Clin Ther. 2005. PMID: 15978306 Clinical Trial.
-
Evaluation of the efficacy and tolerability of the combination delapril plus indapamide in the treatment of mild to moderate essential hypertension: a randomised, multicentre, controlled study.J Hum Hypertens. 2003 Feb;17(2):139-46. doi: 10.1038/sj.jhh.1001514. J Hum Hypertens. 2003. PMID: 12574793 Clinical Trial.
-
Candesartan cilexetil plus hydrochlorothiazide combination: a review of its use in hypertension.Drugs. 2002;62(5):787-816. doi: 10.2165/00003495-200262050-00006. Drugs. 2002. PMID: 11929332 Review.
Cited by
-
Self-Assembly of Angiotensin-Converting Enzyme Inhibitors Captopril and Lisinopril and Their Crystal Structures.Langmuir. 2021 Aug 3;37(30):9170-9178. doi: 10.1021/acs.langmuir.1c01340. Epub 2021 Jul 22. Langmuir. 2021. PMID: 34292730 Free PMC article.
-
Efficacy and safety of delapril/indapamide compared to different ACE-inhibitor/hydrochlorothiazide combinations: a meta-analysis.Int J Gen Med. 2012;5:725-34. doi: 10.2147/IJGM.S35220. Epub 2012 Aug 29. Int J Gen Med. 2012. PMID: 23049265 Free PMC article.
-
Delapril plus indapamide: a review of the combination in the treatment of hypertension.Clin Drug Investig. 2007;27(6):367-80. doi: 10.2165/00044011-200727060-00001. Clin Drug Investig. 2007. PMID: 17506588 Review.
References
-
- Collins R, Peto R, MacMahon S. Blood pressure, stroke, and coronary heart disease: Part 2, Short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. - PubMed
-
- Staessen J.A, Wang J.G, Thijs L. Cardiovascular protection and blood pressure reduction: A meta-analysis. Lancet. 2001;358:1305–1315. - PubMed
-
- Guidelines Subcommittee 1999 World Health Organization–International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens. 1999;17:151–183. - PubMed
-
- Hansson L, Zanchetti A, Carruthers S.G, for the HOT Study Group Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. - PubMed
-
- Mancia G, Carugo S, Grassi G. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control: Data from the PAMELA population. Pressioni Arteriose Monitorate E Loro Associazioni. Hypertension. 2002;39:744–749. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

