Comparison of beraprost and ticlopidine in Chinese patients with chronic peripheral arterial occlusion: a multicenter, single-blind, randomized, controlled study
- PMID: 24944399
- PMCID: PMC4053019
- DOI: 10.1016/S0011-393X(03)00125-5
Comparison of beraprost and ticlopidine in Chinese patients with chronic peripheral arterial occlusion: a multicenter, single-blind, randomized, controlled study
Abstract
Background: Chronic peripheral arterial occlusion (CPAO) is a progressive disease that is associated with a variety of symptoms, the 4 most common being a sensation of coolness in the limbs, intermittent claudication (in which pain occurs on walking), limb pain (which occurs spontaneously at rest), and ischemic leg ulcers. Beraprost sodium is an oral prostaglandin I2 analogue that may ameliorate these symptoms.
Objective: The aim of this study was to compare the efficacy and tolerability of beraprost sodium and ticlopidine hydrochloride in the treatment of patients with CPAO in China.
Methods: In this multicenter, single-blind, controlled study, patients with CPAO were randomly assigned to receive beraprost 120-μg tablet TID or ticlopidine 500-mg tablet BID, both administered orally. The clinical efficacy of the drugs was assessed using the 4 main symptoms of CPAO. Ankle-brachial index (ABI) also was measured as a clinical pharmacologic procedure. Adverse events were assessed throughout the study.
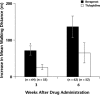
Results: A total of 124 patients (96 men, 28 women; mean [SD] age, 65 [12] years) were enrolled in 3 hospitals. Data from 119 patients (93 men, 26 women; mean [SD] age, 65 [12] years) were included in the efficacy analysis (64 and 55 patients in the beraprost and ticlopidine groups, respectively). Although all 4 symptoms of CPAO were ameliorated after 3 and 6 weeks of treatment with both drugs, only the cool sensation was significantly improved with beraprost compared with ticlopidine at 6 weeks (P<0.05). ABI was significantly increased with both beraprost and ticlopidine at 6 weeks versus baseline (P<0.001 and P<0.01, respectively), suggesting that this pharmacologic action may have led to their beneficial effect on various symptoms. The tolerability analysis included 123 patients (65 and 58 patients in the beraprost and ticlopidine groups, respectively). The numbers of patients who (1) experienced adverse events (AEs), (2) experienced adverse drug reactions, and (3) withdrew due to AEs were significantly smaller in the beraprost group than in the ticlopidine group (P<0.001, P<0.05, and P<0.05, respectively).
Conclusions: In this study population of patients with CPAO, beraprost ameliorated cool sensation in the limbs, intermittent claudication, limb pain, and ischemic/leg ulcers. Beraprost was more efficacious in relieving CPAO symptoms and was better tolerated than ticlopidine. Beraprost may be useful for the treatment of patients with CPAO, but more studies are needed.
Keywords: Beraprost; peripheral arterial occlusion; prostaglandin I2 analogue; ticlopidine.
Figures

References
-
- Itoh M, Mishima Y. Arteriosclerosis obliterans. Geriatr Med. 1995;33:875–880.
-
- Virgolini I, Fitscha P, Linet O.I. A double blind placebo controlled trial of intravenous prostacyclin (PGI2) in 108 patients with ischaemic peripheral vascular disease. Prostaglandins. 1990;39:657–664. - PubMed
-
- McNamara D.B, Champion H.C, Kadowitz P.J. Pharmacologic management of peripheral vascular disease. Surg Clin North Am. 1998;78:447–464. - PubMed
-
- Weitz J.I, Byrne J, Clagett G.P. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996;94:3026–3049. - PubMed
-
- Hiatt W.R. New treatment options in intermittent claudication: The US experience. Int J Clin Pract Suppl. 2001:20–27. - PubMed
LinkOut - more resources
Full Text Sources

