Discovery and Synthesis of C-Nucleosides as Potential New Anti-HCV Agents
- PMID: 24944743
- PMCID: PMC4060939
- DOI: 10.1021/ml500077j
Discovery and Synthesis of C-Nucleosides as Potential New Anti-HCV Agents
Abstract
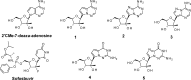
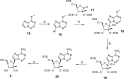
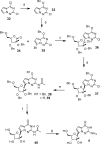
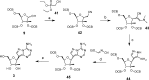
Nucleoside analogues have long been recognized as prospects for the discovery of direct acting antivirals (DAAs) to treat hepatitis C virus because they have generally exhibited cross-genotype activity and a high barrier to resistance. C-Nucleosides have the potential for improved metabolism and pharmacokinetic properties over their N-nucleoside counterparts due to the presence of a strong carbon-carbon glycosidic bond and a non-natural heterocyclic base. Three 2'CMe-C-adenosine analogues and two 2'CMe-guanosine analogues were synthesized and evaluated for their anti-HCV efficacy. The nucleotide triphosphates of four of these analogues were found to inhibit the NS5B polymerase, and adenosine analogue 1 was discovered to have excellent pharmacokinetic properties demonstrating the potential of this drug class.
Keywords: C-Nucleoside; HCV; NS5B polymerase.
Figures


References
-
- Centers for Disease Control and Prevention. Hepatitis C Information for Health Professionals.http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm (accessed October 11, 2013).
-
- Lavanchy D. The Global Burden of Hepatitis C. Liver Int. 2009, 74–81. - PubMed
-
- Poordad F.; Dieterich D. Treating Hepatitis C: Current Standard of Care and Emerging Direct-Acting Antiviral Agents. J. Viral Hepatitis 2012, 19, 449–524. - PubMed
-
- Pawlotsky J.-M. Hepatitis C Virus: Standard-of-Care Treatment. Adv. Pharmacol. 2013, 67, 169–215. - PubMed
-
- US Food and Drug Administration. Viral Hepatitis Therapies. http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm151494... (accessed December 23, 2013).
LinkOut - more resources
Full Text Sources
Other Literature Sources

