Circadian control of glucose metabolism
- PMID: 24944897
- PMCID: PMC4060304
- DOI: 10.1016/j.molmet.2014.03.002
Circadian control of glucose metabolism
Abstract
The incidence of obesity and type 2 diabetes mellitus (T2DM) has risen to epidemic proportions. The pathophysiology of T2DM is complex and involves insulin resistance, pancreatic β-cell dysfunction and visceral adiposity. It has been known for decades that a disruption of biological rhythms (which happens the most profoundly with shift work) increases the risk of developing obesity and T2DM. Recent evidence from basal studies has further sparked interest in the involvement of daily rhythms (and their disruption) in the development of obesity and T2DM. Most living organisms have molecular clocks in almost every tissue, which govern rhythmicity in many domains of physiology, such as rest/activity rhythms, feeding/fasting rhythms, and hormonal secretion. Here we present the latest research describing the specific role played by the molecular clock mechanism in the control of glucose metabolism and speculate on how disruption of these tissue clocks may lead to the disturbances in glucose homeostasis.
Keywords: Autonomic nervous system; Circadian rhythm; Diabetes; Glucose; Hypothalamus.
Figures
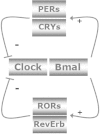
References
-
- Reppert S.M., Weaver D.R. Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology. 2001;63:647–676. - PubMed
-
- Lucas R.J., Lall G.S., Allen A.E., Brown T.M. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Progress in Brain Research. 2012;199:1–18. - PubMed
-
- Balsalobre A., Damiola F., Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

