Rictor/mTORC2 facilitates central regulation of energy and glucose homeostasis
- PMID: 24944899
- PMCID: PMC4060224
- DOI: 10.1016/j.molmet.2014.01.014
Rictor/mTORC2 facilitates central regulation of energy and glucose homeostasis
Abstract
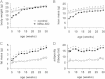
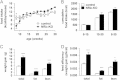
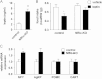
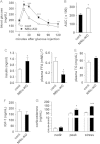
Insulin signaling in the central nervous system (CNS) regulates energy balance and peripheral glucose homeostasis. Rictor is a key regulatory/structural subunit of the mTORC2 complex and is required for hydrophobic motif site phosphorylation of Akt at serine 473. To examine the contribution of neuronal Rictor/mTORC2 signaling to CNS regulation of energy and glucose homeostasis, we utilized Cre-LoxP technology to generate mice lacking Rictor in all neurons, or in either POMC or AgRP expressing neurons. Rictor deletion in all neurons led to increased fat mass and adiposity, glucose intolerance and behavioral leptin resistance. Disrupting Rictor in POMC neurons also caused obesity and hyperphagia, fasting hyperglycemia and pronounced glucose intolerance. AgRP neuron specific deletion did not impact energy balance but led to mild glucose intolerance. Collectively, we show that Rictor/mTORC2 signaling, especially in POMC-expressing neurons, is important for central regulation of energy and glucose homeostasis.
Keywords: AgRP neurons; CNS insulin; Energy balance; Food intake; Obesity; POMC neurons; Rictor; mTORC2.
Figures



References
-
- Bae S.S., Cho H., Mu J., Birnbaum M.J. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. Journal of Biological Chemistry. 2003;278:49530–49536. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

