Gd3+ and calcium sensitive, sodium leak currents are features of weak membrane-glass seals in patch clamp recordings
- PMID: 24945283
- PMCID: PMC4063719
- DOI: 10.1371/journal.pone.0098808
Gd3+ and calcium sensitive, sodium leak currents are features of weak membrane-glass seals in patch clamp recordings
Abstract
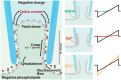
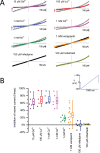
The properties of leaky patch currents in whole cell recording of HEK-293T cells were examined as a means to separate these control currents from expressed sodium and calcium leak channel currents from snail NALCN leak channels possessing both sodium (EKEE) and calcium (EEEE) selectivity filters. Leak currents were generated by the weakening of gigaohm patch seals by artificial membrane rupture using the ZAP function on the patch clamp amplifier. Surprisingly, we found that leak currents generated from the weakened membrane/glass seal can be surprisingly stable and exhibit behavior that is consistent with a sodium leak current derived from an expressible channel. Leaky patch currents differing by 10 fold in size were similarly reduced in size when external sodium ions were replaced with the large monovalent ion NMDG+. Leaky patch currents increased when external Ca2+ (1.2 mM) was lowered to 0.1 mM and were inhibited (>40% to >90%) with 10 µM Gd3+, 100 µM La3+, 1 mM Co2+ or 1 mM Cd2+. Leaky patch currents were relatively insensitive (<30%) to 1 mM Ni2+ and exhibited a variable amount of block with 1 mM verapamil and were insensitive to 100 µM mibefradil or 100 µM nifedipine. We hypothesize that the rapid changes in leak current size in response to changing external cations or drugs relates to their influences on the membrane seal adherence and the electro-osmotic flow of mobile cations channeling in crevices of a particular pore size in the interface between the negatively charged patch electrode and the lipid membrane. Observed sodium leak conductance currents in weak patch seals are reproducible between the electrode glass interface with cell membranes, artificial lipid or Sylgard rubber.
Conflict of interest statement
Figures


References
-
- Thomas P, Smart TG (2005) HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods 51: 187–200. - PubMed
-
- Shaw G, Morse S, Ararat M, Graham FL (2002) Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J 16: 869–871. - PubMed
-
- Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36: 59–74. - PubMed
-
- Gunthorpe MJ, Smith GD, Davis JB, Randall AD (2001) Characterisation of a human acid-sensing ion channel (hASIC1a) endogenously expressed in HEK293 cells. Pflugers Arch 442: 668–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

