Digital genotyping of macrosatellites and multicopy genes reveals novel biological functions associated with copy number variation of large tandem repeats
- PMID: 24945355
- PMCID: PMC4063668
- DOI: 10.1371/journal.pgen.1004418
Digital genotyping of macrosatellites and multicopy genes reveals novel biological functions associated with copy number variation of large tandem repeats
Abstract
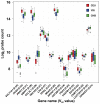
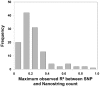
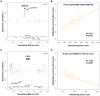
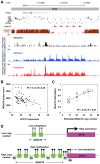
Tandem repeats are common in eukaryotic genomes, but due to difficulties in assaying them remain poorly studied. Here, we demonstrate the utility of Nanostring technology as a targeted approach to perform accurate measurement of tandem repeats even at extremely high copy number, and apply this technology to genotype 165 HapMap samples from three different populations and five species of non-human primates. We observed extreme variability in copy number of tandemly repeated genes, with many loci showing 5-10 fold variation in copy number among humans. Many of these loci show hallmarks of genome assembly errors, and the true copy number of many large tandem repeats is significantly under-represented even in the high quality 'finished' human reference assembly. Importantly, we demonstrate that most large tandem repeat variations are not tagged by nearby SNPs, and are therefore essentially invisible to SNP-based GWAS approaches. Using association analysis we identify many cis correlations of large tandem repeat variants with nearby gene expression and DNA methylation levels, indicating that variations of tandem repeat length are associated with functional effects on the local genomic environment. This includes an example where expansion of a macrosatellite repeat is associated with increased DNA methylation and suppression of nearby gene expression, suggesting a mechanism termed "repeat induced gene silencing", which has previously been observed only in transgenic organisms. We also observed multiple signatures consistent with altered selective pressures at tandemly repeated loci, suggesting important biological functions. Our studies show that tandemly repeated loci represent a highly variable fraction of the genome that have been systematically ignored by most previous studies, copy number variation of which can exert functionally significant effects. We suggest that future studies of tandem repeat loci will lead to many novel insights into their role in modulating both genomic and phenotypic diversity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. (2001) Initial sequencing and analysis of the human genome. Nature 409: 860–921. - PubMed
-
- Sharp AJ, Itsara A, Cheng Z, Alkan C, Schwartz S, et al. (2007) Optimal design of oligonucleotide microarrays for measurement of DNA copy-number. Hum Mol Genet 16: 2770–2779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

