Advanced CLARITY for rapid and high-resolution imaging of intact tissues
- PMID: 24945384
- PMCID: PMC4096681
- DOI: 10.1038/nprot.2014.123
Advanced CLARITY for rapid and high-resolution imaging of intact tissues
Abstract
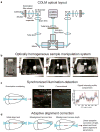
CLARITY is a method for chemical transformation of intact biological tissues into a hydrogel-tissue hybrid, which becomes amenable to interrogation with light and macromolecular labels while retaining fine structure and native biological molecules. This emerging accessibility of information from large intact samples has created both new opportunities and new challenges. Here we describe protocols spanning multiple dimensions of the CLARITY workflow, ranging from simple, reliable and efficient lipid removal without electrophoretic instrumentation (passive CLARITY) to optimized objectives and integration with light-sheet optics (CLARITY-optimized light-sheet microscopy (COLM)) for accelerating data collection from clarified samples by several orders of magnitude while maintaining or increasing quality and resolution. The entire protocol takes from 7-28 d to complete for an adult mouse brain, including hydrogel embedding, full lipid removal, whole-brain antibody staining (which, if needed, accounts for 7-10 of the days), and whole-brain high-resolution imaging; timing within this window depends on the choice of lipid removal options, on the size of the tissue, and on the number and type of immunostaining rounds performed. This protocol has been successfully applied to the study of adult mouse, adult zebrafish and adult human brains, and it may find many other applications in the structural and molecular analysis of large assembled biological systems.
Conflict of interest statement
Competing Interests Statement: None.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

