Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil's disease
- PMID: 24945433
- PMCID: PMC4170969
- DOI: 10.1111/iep.12085
Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil's disease
Abstract
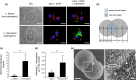
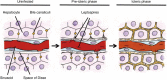
Weil's disease, the most severe form of leptospirosis, is characterized by jaundice, haemorrhage and renal failure. The mechanisms of jaundice caused by pathogenic Leptospira remain unclear. We therefore aimed to elucidate the mechanisms by integrating histopathological changes with serum biochemical abnormalities during the development of jaundice in a hamster model of Weil's disease. In this work, we obtained three-dimensional images of infected hamster livers using scanning electron microscope together with freeze-cracking and cross-cutting methods for sample preparation. The images displayed the corkscrew-shaped bacteria, which infiltrated the Disse's space, migrated between hepatocytes, detached the intercellular junctions and disrupted the bile canaliculi. Destruction of bile canaliculi coincided with the elevation of conjugated bilirubin, aspartate transaminase and alkaline phosphatase levels in serum, whereas serum alanine transaminase and γ-glutamyl transpeptidase levels increased slightly, but not significantly. We also found in ex vivo experiments that pathogenic, but not non-pathogenic leptospires, tend to adhere to the perijunctional region of hepatocyte couplets isolated from hamsters and initiate invasion of the intercellular junction within 1 h after co-incubation. Our results suggest that pathogenic leptospires invade the intercellular junctions of host hepatocytes, and this invasion contributes in the disruption of the junction. Subsequently, bile leaks from bile canaliculi and jaundice occurs immediately. Our findings revealed not only a novel pathogenicity of leptospires, but also a novel mechanism of jaundice induced by bacterial infection.
Keywords: Leptospira; bacterial invasion; cell junction; hepatocyte couplet; jaundice; scanning electron microscope.
© 2014 The Authors. International Journal of Experimental Pathology © 2014 International Journal of Experimental Pathology.
Figures



References
-
- Anderson JM. Leaky junctions and cholestasis: a tight correlation. Gastroenterology. 1996;110:1662–1665. - PubMed
-
- Arean VM. Studies on the pathogenesis of leptospirosis. II. A clinicopathologic evaluation of hepatic and renal function in experimental leptospiral infections. Lab. Invest. 1962b;11:273–288. - PubMed
-
- Balkovetz DF, Katz J. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 2003;5:613–619. - PubMed
-
- Ballard SA, Williamson M, Adler B, Vinh T, Faine S. Interaction of virulent and avirulent leptospires with primary cultures of renal epithelial cells. J. Med. Microbiol. 1986;21:59–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

