Stabilizing rescued surface-localized δf508 CFTR by potentiation of its interaction with Na(+)/H(+) exchanger regulatory factor 1
- PMID: 24945463
- PMCID: PMC4081048
- DOI: 10.1021/bi401263h
Stabilizing rescued surface-localized δf508 CFTR by potentiation of its interaction with Na(+)/H(+) exchanger regulatory factor 1
Abstract
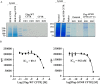
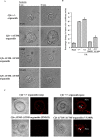
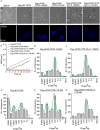
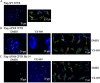
Cystic fibrosis (CF) is a recessive genetic disease caused by mutations in CFTR, a plasma-membrane-localized anion channel. The most common mutation in CFTR, deletion of phenylalanine at residue 508 (ΔF508), causes misfolding of CFTR resulting in little or no protein at the plasma membrane. The CFTR corrector VX-809 shows promise for treating CF patients homozygous for ΔF508. Here, we demonstrate the significance of protein-protein interactions in enhancing the stability of the ΔF508 CFTR mutant channel protein at the plasma membrane. We determined that VX-809 prolongs the stability of ΔF508 CFTR at the plasma membrane. Using competition-based assays, we demonstrated that ΔF508 CFTR interacts poorly with Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) compared to wild-type CFTR, and VX-809 significantly increased this binding affinity. We conclude that stabilized CFTR-NHERF1 interaction is a determinant of the functional efficiency of rescued ΔF508 CFTR. Our results demonstrate the importance of macromolecular-complex formation in stabilizing rescued mutant CFTR at the plasma membrane and suggest this to be foundational for the development of a new generation of effective CFTR-corrector-based therapeutics.
Figures

References
-
- Rowe S. M.; Miller S.; Sorscher E. J. (2005) Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001. - PubMed
-
- Rowntree R. K.; Harris A. (2003) The phenotypic consequences of CFTR mutations. Ann. Hum. Genet. 67, 471–485. - PubMed
-
- Kerem B.; Rommens J. M.; Buchanan J. A.; Markiewicz D.; Cox T. K.; Chakravarti A.; Buchwald M.; Tsui L. C. (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245, 1073–1080. - PubMed
-
- Bobadilla J. L.; Macek M. Jr.; Fine J. P.; Farrell P. M. (2002) Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum. Mutat. 19, 575–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

