Norepinephrine controls astroglial responsiveness to local circuit activity
- PMID: 24945771
- PMCID: PMC4080721
- DOI: 10.1016/j.neuron.2014.04.038
Norepinephrine controls astroglial responsiveness to local circuit activity
Abstract
Astrocytes perform crucial supportive functions, including neurotransmitter clearance, ion buffering, and metabolite delivery. They can also influence blood flow and neuronal activity by releasing gliotransmitters in response to intracellular Ca(2+) transients. However, little is known about how astrocytes are engaged during different behaviors in vivo. Here we demonstrate that norepinephrine primes astrocytes to detect changes in cortical network activity. We show in mice that locomotion triggers simultaneous activation of astrocyte networks in multiple brain regions. This global stimulation of astrocytes was inhibited by alpha-adrenoceptor antagonists and abolished by depletion of norepinephrine from the brain. Although astrocytes in visual cortex of awake mice were rarely engaged when neurons were activated by light stimulation alone, pairing norepinephrine release with light stimulation markedly enhanced astrocyte Ca(2+) signaling. Our findings indicate that norepinephrine shifts the gain of astrocyte networks according to behavioral state, enabling astrocytes to respond to local changes in neuronal activity.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
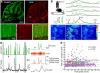
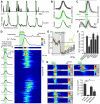


Comment in
-
Glia: noradrenaline arouses astrocytes.Nat Rev Neurosci. 2014 Aug;15(8):494. doi: 10.1038/nrn3788. Epub 2014 Jul 9. Nat Rev Neurosci. 2014. PMID: 25005483 No abstract available.
References
-
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. - PMC - PubMed
-
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 2011;14:1276–1284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

