The influence of the CYP2C19*10 allele on clopidogrel activation and CYP2C19*2 genotyping
- PMID: 24945780
- PMCID: PMC4090277
- DOI: 10.1097/FPC.0000000000000068
The influence of the CYP2C19*10 allele on clopidogrel activation and CYP2C19*2 genotyping
Erratum in
- Pharmacogenet Genomics. 2014 Dec;24(12):622
Abstract
Background/objectives: The polymorphic hepatic enzyme CYP2C19 catalyzes the metabolism of clinically important drugs such as clopidogrel, proton-pump inhibitors, and others and clinical pharmacogenetic testing for clopidogrel is increasingly common. The CYP2C19*10 single-nucleotide polymorphism (SNP) is located 1 bp upstream the CYP2C19*2 SNP. Despite the low frequency of the CYP2C19*10 allele, its impact on metabolism of CYP2C19 substrates and CYP2C19*2 genotyping makes it an important SNP to consider for pharmacogenetic testing of CYP2C19. However, the effect of the CYP2C19*10 allele on clopidogrel metabolism has not been explored to date.
Methods: We measured the enzymatic activity of the CYP2C19.10 protein against clopidogrel. DNA samples from two clinical studies were genotyped for CYP2C19*2 and *10 by pyrosequencing genotyping method.
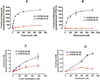
Results: The catalytic activity of CYP2C19.10 in the biotransformation of clopidogrel and 2-oxo-clopidogrel was significantly decreased relative to the wild-type CYP2C19.1B. We also reported that the CYP2C19*10 SNP interferes with the CYP2C19*2 TaqMan genotyping assay, resulting in miscalling of CYP2C19*10/*2 as CYP2C19*2/*2.
Conclusions: Our data provide evidence that CYP2C19.10 variant partially metabolizes clopidogrel and 2-oxo-clopidogrel, and the presence of CYP2C19*10 allele affects the CY2C19*2 TaqMan genotyping assay and results in misclassification of CYP2C19*10/*2 as CYP2C19*2/*2.
Conflict of interest statement
Conflict of Interest
There are no conflicts of interest declared.
Figures
References
-
- Blaisdell J, Mohrenweiser H, Jackson J, Ferguson S, Coulter S, Chanas B, et al. Identification and functional characterization of new potentially defective alleles of human CYP2C19. Pharmacogenetics. 2002;12:703–711. - PubMed
-
- Wang H, Kim RA, Sun D, Gao Y, Wang H, Zhu J, et al. Evaluation of the effects of 18 non-synonymous single-nucleotide polymorphisms of CYP450 2C19 on in vitro drug inhibition potential by a fluorescence-based high-throughput assay. Xenobiotica. 2011;41(9):826–835. - PubMed
-
- Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, et al. Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90(2):328–332. - PMC - PubMed
-
- Rasmussen H, Werge T. Misclassification of allele CYP2C19*10 as CY2C19*2 by a commonly used PCR-RFLP procedure. GenetTest. 2008;12(1):57–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

