Nfatc2 and Tob1 have non-overlapping function in T cell negative regulation and tumorigenesis
- PMID: 24945807
- PMCID: PMC4063948
- DOI: 10.1371/journal.pone.0100629
Nfatc2 and Tob1 have non-overlapping function in T cell negative regulation and tumorigenesis
Abstract
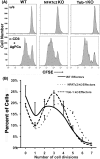
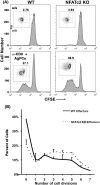
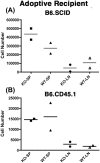
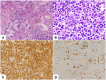
Nfatc2 and Tob1 are intrinsic negative regulators of T cell activation. Nfatc2-deficient and Tob1-deficient T cells show reduced thresholds of activation; however, whether these factors have independent or overlapping roles in negative regulation of T cell responses has not been previously examined. Here, we show that Nfatc2 knockout (KO) but not Tob1 KO mice have age-associated accumulation of persistently activated T cells in vivo and expansion of the CD44+ memory cell compartment and age-associated lymphocytic infiltrates in visceral organs, without significant changes in numbers of CD4+CD25+Foxp3+ regulatory T cells (Treg). In vitro, CD4+CD25- "conventional" T cells (Tconvs) from both KO strains showed greater proliferation than wild type (WT) Tconvs. However, while Tregs from Nfatc2 KO mice retained normal suppressive function, Tregs from Tob1 KOs had enhanced suppressive activity. Nfatc2 KO Tconvs expanded somewhat more rapidly than WT Tconvs under conditions of homeostatic proliferation, but their accelerated growth capacity was negated, at least acutely, in a lymphoreplete environment. Finally, Nfatc2 KO mice developed a previously uncharacterized increase in B-cell malignancies, which was not accelerated by the absence of Tob1. The data thus support the prevailing hypothesis that Nfatc2 and Tob1 are non-redundant regulators of lymphocyte homeostasis.
Conflict of interest statement
Figures



Similar articles
-
Increased immunosuppressive function of CD4(+)CD25(+)Foxp3(+)GITR+ T regulatory cells from NFATc2((-/-)) mice controls allergen-induced experimental asthma.Immunobiology. 2012 Sep;217(9):905-11. doi: 10.1016/j.imbio.2012.01.004. Epub 2012 Jan 11. Immunobiology. 2012. PMID: 22317750
-
Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4+CD25+Foxp3+ and CD4+CD25-Foxp3+ T cells.J Transl Med. 2019 Jul 9;17(1):219. doi: 10.1186/s12967-019-1967-3. J Transl Med. 2019. PMID: 31288845 Free PMC article.
-
Eos Is Redundant for Regulatory T Cell Function but Plays an Important Role in IL-2 and Th17 Production by CD4+ Conventional T Cells.J Immunol. 2015 Jul 15;195(2):553-63. doi: 10.4049/jimmunol.1500627. Epub 2015 Jun 10. J Immunol. 2015. PMID: 26062998 Free PMC article.
-
The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road.J Cell Physiol. 2007 Jun;211(3):590-7. doi: 10.1002/jcp.21001. J Cell Physiol. 2007. PMID: 17311282 Review.
-
IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells.Front Biosci. 2008 Jan 1;13:1440-6. doi: 10.2741/2773. Front Biosci. 2008. PMID: 17981641 Review.
Cited by
-
Rare diseases of epigenetic origin: Challenges and opportunities.Front Genet. 2023 Feb 6;14:1113086. doi: 10.3389/fgene.2023.1113086. eCollection 2023. Front Genet. 2023. PMID: 36814905 Free PMC article. Review.
-
Transcriptomic Analysis Exploring the Molecular Mechanisms of Hanchuan Zupa Granules in Alleviating Asthma in Rat.Evid Based Complement Alternat Med. 2021 Jul 2;2021:5584099. doi: 10.1155/2021/5584099. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34285702 Free PMC article.
-
Differential genetic and functional background in inflammatory bowel disease phenotypes of a Greek population: a systems bioinformatics approach.Gut Pathog. 2019 Jun 15;11:31. doi: 10.1186/s13099-019-0312-y. eCollection 2019. Gut Pathog. 2019. PMID: 31249629 Free PMC article.
-
NFAT1 transcription factor regulates cell cycle progression and cyclin E expression in B lymphocytes.Cell Cycle. 2016 Sep;15(17):2346-59. doi: 10.1080/15384101.2016.1203485. Epub 2016 Jul 11. Cell Cycle. 2016. PMID: 27399331 Free PMC article.
-
Cell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old player.Cell Death Dis. 2016 Apr 21;7(4):e2199. doi: 10.1038/cddis.2016.97. Cell Death Dis. 2016. PMID: 27100893 Free PMC article. Review.
References
-
- Kiani A, Rao A, Aramburu J (2000) Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity 12: 359–372. - PubMed
-
- Sundrud MS, Rao A (2007) New twists of T cell fate: control of T cell activation and tolerance by TGF-beta and NFAT. Curr Opin Immunol 19: 287–293. - PubMed
-
- Macian F, Lopez-Rodriguez C, Rao A (2001) Partners in transcription: NFAT and AP-1. Oncogene 20: 2476–2489. - PubMed
-
- Muller MR, Rao A (2010) NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol 10: 645–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA065493/CA/NCI NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- R01CA72669/CA/NCI NIH HHS/United States
- R21 DK063410/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
- R21DK63410/DK/NIDDK NIH HHS/United States
- R01HL56067/HL/NHLBI NIH HHS/United States
- R01AI081918/AI/NIAID NIH HHS/United States
- P30 CA077598/CA/NCI NIH HHS/United States
- P01CA065493/CA/NCI NIH HHS/United States
- P30CA077598/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

