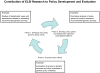
The translational potential of research on the ethical, legal, and social implications of genomics
- PMID: 24946153
- PMCID: PMC4272334
- DOI: 10.1038/gim.2014.74
The translational potential of research on the ethical, legal, and social implications of genomics
Abstract
Federally funded research on the ethical, legal, and social implications (ELSI) of genomics includes a programmatic charge to consider policy-relevant questions and to communicate findings in venues that help inform the policy-making process. In addressing this goal, investigators must consider the range of policies that are relevant to human genetics; how foundational research in bioethics, law, and the social sciences might inform those policies; and the potential professional issues that this translational imperative raises for ELSI investigators. We review these questions in light of experiences from a consortium of federally funded Centers of Excellence in ELSI Research, and offer a set of policy recommendations for program design and evaluation of ELSI research. We conclude that it would be a mistake to require that ELSI research programs demonstrate a direct impact on science or health policy; however, ELSI researchers can take steps to increase the relevance of their work to policy makers. Similarly, funders of ELSI research who are concerned with facilitating policy development can help by building cross-disciplinary translational research capacities, and universities can take steps to make policy-relevant research more rewarding for scholars in the humanities, social sciences, and law.
Figures
References
-
- Juengst ET. Self-critical federal science? The ethics experiment within the U.S. Human Genome Project. [Accessed March 15, 2014];Soc Philos Policy. 1996 Summer;13(2):63–95. Department of Health and Human Services RFA-HG-12-005 Specialized Centers of Excellence in ELSI Research (CEER) (P50). http://grants.nih.gov/grants/guide/rfa-files/RFA-HG-12-005.html. - PubMed
-
- Hanna KE. The Ethical Legal and Social Implications Program of the National Center for Human Genome Research: A Missed Opportunity? In: Bulger R, Bobby E, Fineberg H, editors. Society's Choices: Social and Ethical Decision-making in Biomedicine. Washington, DC: National Academies Press; 1995. pp. 432–58. - PubMed
-
- De Leeuw E. Policies for Health The effectiveness of their development, adoption, and implementation. In: McQueen D, Jones CM, editors. Global Perspectives on Health Promotion Effectiveness. Springer; New York: 2007. pp. 51–66. Chapter 5.
-
- Genetic Information Nondiscrimination Act (GINA) [Accessed March 15, 2014]; http://www.gpo.gov/fdsys/pkg/PLAW-110publ233/content-detail.html.
-
- US Department of Health and Human Services. Federal Policy for the Protection of Human Subjects (‘Common Rule’) [Accessed March 15, 2014]; http://www.hhs.gov/ohrp/humansubjects/commonrule/
Publication types
MeSH terms
Grants and funding
- P50HG007257/HG/NHGRI NIH HHS/United States
- P50HG003374/HG/NHGRI NIH HHS/United States
- P50HG004488/HG/NHGRI NIH HHS/United States
- P50 HG004488/HG/NHGRI NIH HHS/United States
- P50HG003390/HG/NHGRI NIH HHS/United States
- P50 HG004487/HG/NHGRI NIH HHS/United States
- P50 HG003391/HG/NHGRI NIH HHS/United States
- P50 HG003374/HG/NHGRI NIH HHS/United States
- P50 HG003390/HG/NHGRI NIH HHS/United States
- P50 HG007257/HG/NHGRI NIH HHS/United States
- P50HG003391/HG/NHGRI NIH HHS/United States
- P50HG004487/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources