A neurocentric perspective on glioma invasion
- PMID: 24946761
- PMCID: PMC5304245
- DOI: 10.1038/nrn3765
A neurocentric perspective on glioma invasion
Abstract
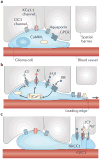
Malignant gliomas are devastating tumours that frequently kill patients within 1 year of diagnosis. The major obstacle to a cure is diffuse invasion, which enables tumours to escape complete surgical resection and chemo- and radiation therapy. Gliomas use the same tortuous extracellular routes of migration that are travelled by immature neurons and stem cells, frequently using blood vessels as guides. They repurpose ion channels to dynamically adjust their cell volume to accommodate to narrow spaces and breach the blood-brain barrier through disruption of astrocytic endfeet, which envelop blood vessels. The unique biology of glioma invasion provides hitherto unexplored brain-specific therapeutic targets for this devastating disease.
Figures



References
-
- Central Brain Tumor Registry of the United States. CBTRUS Statistical report: primary brain and central nervous system tumors diagnosed in the United States2004–2006. CBTRUS. 2010 [online], http://www.cbtrus.org/2010-NPCR-SEER/CBTRUS-WEBREPORT-Final-3-2-10.pdf.
-
- Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia: preliminary report. JAMA. 1928;90:823–825.
-
- Hou LC, Veeravagu A, Hsu AR, Tse VC. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20:E5. - PubMed
-
- Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. This paper shows that gliomas can be molecularly classified into four subtypes (pro-neural, neural, classical and mesenchymal) that respond differently to therapeutic intervention. - PMC - PubMed
-
- Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nature Rev Cancer. 2010;10:319–331. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS082851/NS/NINDS NIH HHS/United States
- R01 NS031234/NS/NINDS NIH HHS/United States
- F31 NS074597/NS/NINDS NIH HHS/United States
- 5R01NS031234/NS/NINDS NIH HHS/United States
- F31NS074597/NS/NINDS NIH HHS/United States
- 5R01‑NS052634/NS/NINDS NIH HHS/United States
- R01 NS052634/NS/NINDS NIH HHS/United States
- R01 NS036692/NS/NINDS NIH HHS/United States
- 2R01‑NS036692/NS/NINDS NIH HHS/United States
- 1R01‑NS082851/NS/NINDS NIH HHS/United States
- F31 NS073181/NS/NINDS NIH HHS/United States
- F31NS073181/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

