Combining AKT inhibition with chloroquine and gefitinib prevents compensatory autophagy and induces cell death in EGFR mutated NSCLC cells
- PMID: 24946858
- PMCID: PMC4148097
- DOI: 10.18632/oncotarget.2017
Combining AKT inhibition with chloroquine and gefitinib prevents compensatory autophagy and induces cell death in EGFR mutated NSCLC cells
Abstract
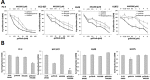
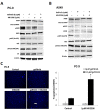
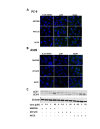
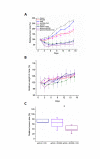
Although non-small cell lung cancer (NSCLC) patients with EGFR mutation positive (EGFR M+) tumors initially respond well to EGFR tyrosine kinase inhibitor (TKI) monotherapy, the responses are usually incomplete. In this study we show that AKT inhibition, most importantly AKT2 inhibition, synergises with EGFR TKI inhibition to increase cell killing in EGFR M+ NSCLC cells. However, our data also suggest that the synergistic pro-apoptotic effects may be stunted due to a prosurvival autophagy response induced by AKT inhibition. Consequently, inhibiting autophagy with chloroquine significantly enhanced tumor cell death induced by gefitinib and AKT inhibitors in EGFR M+ cells in vitro, and produced greater tumor shrinkage in EGFR M+ xenografts in vivo. Together, our findings suggest that adding chloroquine to EGFR and AKT inhibition has the potential to improve tumor responses in EGFR M+ NSCLC, and that selective targeting of AKT2 may provide a new treatment option in NSCLC.
Figures



References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. Ca-a Cancer Journal for Clinicians. 2011;61(2):69–90. - PubMed
-
- Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. European Respiratory Journal. 2001;18(6):1059–1068. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New England Journal of Medicine. 2004;350(21):2129–2139. - PubMed
-
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13306–13311. - PMC - PubMed
-
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. New England Journal of Medicine. 2005;352(8):786–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

