Direct measurements of SR free Ca reveal the mechanism underlying the transient effects of RyR potentiation under physiological conditions
- PMID: 24947416
- PMCID: PMC4145011
- DOI: 10.1093/cvr/cvu158
Direct measurements of SR free Ca reveal the mechanism underlying the transient effects of RyR potentiation under physiological conditions
Abstract
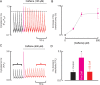
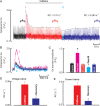
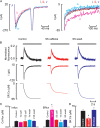
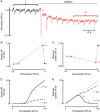
Aims: Most of the calcium that activates contraction is released from the sarcoplasmic reticulum (SR) through the ryanodine receptor (RyR). It is controversial whether activators of the RyR produce a maintained increase in the amplitude of the systolic Ca transient. We therefore aimed to examine the effects of activation of the RyR in large animals under conditions designed to be as physiological as possible while simultaneously measuring SR and cytoplasmic Ca.
Methods and results: Experiments were performed on ventricular myocytes from canine and ovine hearts. Cytoplasmic Ca was measured with fluo-3 and SR Ca with mag-fura-2. Application of caffeine resulted in a brief increase in the amplitude of the systolic Ca transient accompanied by an increase of action potential duration. These effects disappeared with a rate constant of ∼3 s(-1). Similar effects were seen in cells taken from sheep in which heart failure had been induced by rapid pacing. The decrease of Ca transient amplitude was accompanied by a decrease of SR Ca content. During this phase, the maximum (end-diastolic) SR Ca content fell while the minimum systolic increased.
Conclusions: This study shows that, under conditions designed to be as physiological as possible, potentiation of RyR opening has no maintained effect on the systolic Ca transient. This result makes it unlikely that potentiation of the RyR has a maintained role in positive inotropy.
Keywords: Calcium; Ryanodine receptor; Sarcoplasmic reticulum.
© The Author 2014. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Similar articles
-
A novel mechanism of tandem activation of ryanodine receptors by cytosolic and SR luminal Ca2+ during excitation-contraction coupling in atrial myocytes.J Physiol. 2017 Jun 15;595(12):3835-3845. doi: 10.1113/JP273611. Epub 2017 Feb 1. J Physiol. 2017. PMID: 28028837 Free PMC article.
-
Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: ryanodine receptor refractoriness during alternans and fibrillation.Circ Res. 2014 Apr 25;114(9):1410-21. doi: 10.1161/CIRCRESAHA.114.302505. Epub 2014 Feb 25. Circ Res. 2014. PMID: 24568740 Free PMC article.
-
What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis?J Mol Cell Cardiol. 2009 Apr;46(4):474-81. doi: 10.1016/j.yjmcc.2008.12.005. Epub 2008 Dec 25. J Mol Cell Cardiol. 2009. PMID: 19150449 Review.
-
Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure.J Physiol. 2009 Nov 1;587(Pt 21):5197-209. doi: 10.1113/jphysiol.2009.177576. Epub 2009 Sep 7. J Physiol. 2009. PMID: 19736296 Free PMC article.
-
Physiological and pathological modulation of ryanodine receptor function in cardiac muscle.Cell Calcium. 2004 Jun;35(6):583-9. doi: 10.1016/j.ceca.2004.01.012. Cell Calcium. 2004. PMID: 15110148 Review.
Cited by
-
Examination of the Effects of Heterogeneous Organization of RyR Clusters, Myofibrils and Mitochondria on Ca2+ Release Patterns in Cardiomyocytes.PLoS Comput Biol. 2015 Sep 3;11(9):e1004417. doi: 10.1371/journal.pcbi.1004417. eCollection 2015 Sep. PLoS Comput Biol. 2015. PMID: 26335304 Free PMC article.
-
Determinants of Ca2+ release restitution: Insights from genetically altered animals and mathematical modeling.J Gen Physiol. 2020 Nov 2;152(11):e201912512. doi: 10.1085/jgp.201912512. J Gen Physiol. 2020. PMID: 32986800 Free PMC article.
-
Examining Cardiomyocyte Dysfunction Using Acute Chemical Induction of an Ageing Phenotype.Int J Mol Sci. 2019 Dec 27;21(1):197. doi: 10.3390/ijms21010197. Int J Mol Sci. 2019. PMID: 31892165 Free PMC article.
-
Perturbed atrial calcium handling in an ovine model of heart failure: potential roles for reductions in the L-type calcium current.J Mol Cell Cardiol. 2015 Feb;79:169-79. doi: 10.1016/j.yjmcc.2014.11.017. Epub 2014 Nov 22. J Mol Cell Cardiol. 2015. PMID: 25463272 Free PMC article.
-
Systolic [Ca2+ ]i regulates diastolic levels in rat ventricular myocytes.J Physiol. 2017 Aug 15;595(16):5545-5555. doi: 10.1113/JP274366. Epub 2017 Jul 23. J Physiol. 2017. PMID: 28617952 Free PMC article.
References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Meissner G. Regulation of mammalian ryanodine receptors. Front Biosci. 2002;7:d2072–d2080. - PubMed
-
- Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. - PMC - PubMed
-
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FS/10/71/28563/BHF_/British Heart Foundation/United Kingdom
- PG/10/89/28630/BHF_/British Heart Foundation/United Kingdom
- PG/08/078/25593/BHF_/British Heart Foundation/United Kingdom
- FS/12/57/29717/BHF_/British Heart Foundation/United Kingdom
- FS/09/036/27823/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

