Actin enables the antimicrobial action of LL-37 peptide in the presence of microbial proteases
- PMID: 24947511
- PMCID: PMC4132794
- DOI: 10.1074/jbc.M114.579672
Actin enables the antimicrobial action of LL-37 peptide in the presence of microbial proteases
Abstract
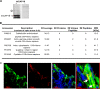
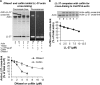
Host defense peptides play an important host-protective role by their microcidal action, immunomodulatory functions, and tissue repair activities. Proteolysis is a common strategy of pathogens used to neutralize host defense peptides. Here, we show that actin, the most abundant structural protein in eukaryotes, binds the LL-37 host defense peptide, protects it from degradation by the proteases of Pseudomonas aeruginosa and Porphyromonas gingivalis, and enables its antimicrobial activity despite the presence of the proteases. Co-localization of LL-37 with extracellular actin was observed in necrotized regions of samples from oral lesions. Competition assays, cross-linking experiments, limited proteolysis, and mass spectrometry revealed that LL-37 binds by specific hydrophobic interactions to the His-40-Lys-50 segment of actin, located in the DNase I binding loop. The integrity of the binding site of both LL-37 and actin is a prerequisite to the binding. Our results demonstrate that actin, presumably released by dead cells and abundant in infected sites, might be utilized by the immune system to enhance spatio-temporal immunity in an attempt to arrest infection and control inflammation.
Keywords: Actin; Antimicrobial Peptide (AMP); Host Defense; Protease; Transglutaminase.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures










Similar articles
-
Saliva enables the antimicrobial activity of LL-37 in the presence of proteases of Porphyromonas gingivalis.Infect Immun. 2009 Dec;77(12):5558-63. doi: 10.1128/IAI.00648-09. Epub 2009 Oct 5. Infect Immun. 2009. PMID: 19805540 Free PMC article.
-
The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium.Am J Respir Cell Mol Biol. 2010 Dec;43(6):692-702. doi: 10.1165/rcmb.2009-0250OC. Epub 2010 Jan 22. Am J Respir Cell Mol Biol. 2010. PMID: 20097832 Free PMC article.
-
Cationic antimicrobial peptides promote microbial mutagenesis and pathoadaptation in chronic infections.PLoS Pathog. 2014 Apr 24;10(4):e1004083. doi: 10.1371/journal.ppat.1004083. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24763694 Free PMC article. Clinical Trial.
-
Antimicrobial proteins and peptides in human lung diseases: A friend and foe partnership with host proteases.Biochimie. 2016 Mar;122:151-68. doi: 10.1016/j.biochi.2015.08.014. Epub 2015 Sep 2. Biochimie. 2016. PMID: 26341472 Review.
-
Impact of LL-37 on anti-infective immunity.J Leukoc Biol. 2005 Apr;77(4):451-9. doi: 10.1189/jlb.0704380. Epub 2004 Nov 29. J Leukoc Biol. 2005. PMID: 15569695 Review.
Cited by
-
Antimicrobial peptides in 2014.Pharmaceuticals (Basel). 2015 Mar 23;8(1):123-50. doi: 10.3390/ph8010123. Pharmaceuticals (Basel). 2015. PMID: 25806720 Free PMC article. Review.
-
Interactions of histatin-3 and histatin-5 with actin.BMC Biochem. 2017 Mar 6;18(1):3. doi: 10.1186/s12858-017-0078-0. BMC Biochem. 2017. PMID: 28264651 Free PMC article.
-
Polymicrobial synergy and dysbiosis in inflammatory disease.Trends Mol Med. 2015 Mar;21(3):172-83. doi: 10.1016/j.molmed.2014.11.004. Epub 2014 Nov 20. Trends Mol Med. 2015. PMID: 25498392 Free PMC article. Review.
-
Histones bundle F-actin filaments and affect actin structure.PLoS One. 2017 Aug 28;12(8):e0183760. doi: 10.1371/journal.pone.0183760. eCollection 2017. PLoS One. 2017. PMID: 28846729 Free PMC article.
-
Mechanisms of Antimicrobial Peptide Resistance in Gram-Negative Bacteria.Antibiotics (Basel). 2015 Mar;4(1):18-41. doi: 10.3390/antibiotics4010018. Antibiotics (Basel). 2015. PMID: 25927010 Free PMC article.
References
-
- Altman H., Steinberg D., Porat Y., Mor A., Fridman D., Friedman M., Bachrach G. (2006) In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J. Antimicrob. Chemother. 58, 198–201 - PubMed
-
- Hilpert K., Volkmer-Engert R., Walter T., Hancock R. E. (2005) High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 23, 1008–1012 - PubMed
-
- Radzishevsky I. S., Rotem S., Bourdetsky D., Navon-Venezia S., Carmeli Y., Mor A. (2007) Improved antimicrobial peptides based on acyl-lysine oligomers. Nat. Biotechnol. 25, 657–659 - PubMed
-
- Hancock R. E., Nijnik A., Philpott D. J. (2012) Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254 - PubMed
-
- Brogden K. A. (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

