Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1
- PMID: 24947528
- PMCID: PMC4103955
- DOI: 10.1161/ATVBAHA.114.303983
Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1
Abstract
Objective: Perivascular adipose tissue (PVAT) expands during obesity, is highly inflamed, and correlates with coronary plaque burden and increased cardiovascular risk. We tested the hypothesis that PVAT contributes to the vascular response to wire injury and investigated the underlying mechanisms.
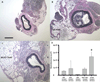
Approach and results: We transplanted thoracic aortic PVAT from donor mice fed a high-fat diet to the carotid arteries of recipient high-fat diet-fed low-density lipoprotein receptor knockout mice. Two weeks after transplantation, wire injury was performed, and animals were euthanized 2 weeks later. Immunohistochemistry was performed to quantify adventitial macrophage infiltration and neovascularization and neointimal lesion composition and size. Transplanted PVAT accelerated neointimal hyperplasia, adventitial macrophage infiltration, and adventitial angiogenesis. The majority of neointimal cells in PVAT-transplanted animals expressed α-smooth muscle actin, consistent with smooth muscle phenotype. Deletion of monocyte chemoattractant protein-1 in PVAT substantially attenuated the effects of fat transplantation on neointimal hyperplasia and adventitial angiogenesis, but not adventitial macrophage infiltration. Conditioned medium from perivascular adipocytes induced potent monocyte chemotaxis in vitro and angiogenic responses in cultured endothelial cells.
Conclusions: These findings indicate that PVAT contributes to the vascular response to wire injury, in part through monocyte chemoattractant protein-1-dependent mechanisms.
Keywords: adipose tissue; hyperplasia.
© 2014 American Heart Association, Inc.
Figures



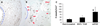

References
-
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. - PubMed
-
- Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. - PubMed
-
- Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, Erbel R, Mohlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. - PubMed
-
- Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

