Oedema-based model for diffuse low-grade gliomas: application to clinical cases under radiotherapy
- PMID: 24947764
- PMCID: PMC6496677
- DOI: 10.1111/cpr.12114
Oedema-based model for diffuse low-grade gliomas: application to clinical cases under radiotherapy
Abstract
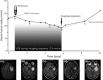
Objectives: Diffuse low-grade gliomas are characterized by slow growth. Despite appropriate treatment, they change inexorably into more aggressive forms, jeopardizing the patient's life. Optimizing treatments, for example with the use of mathematical modelling, could help to prevent tumour regrowth and anaplastic transformation. Here, we present a model of the effect of radiotherapy on such tumours. Our objective is to explain observed delay of tumour regrowth following radiotherapy and to predict its duration.
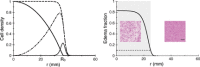
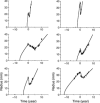
Materials and methods: We have used a migration-proliferation model complemented by an equation describing appearance and draining of oedema. The model has been applied to clinical data of tumour radius over time, for a population of 28 patients.
Results: We were able to show that draining of oedema accounts for regrowth delay after radiotherapy and have been able to fit the clinical data in a robust way. The model predicts strong correlation between high proliferation coefficient and low progression-free gain of lifetime, due to radiotherapy among the patients, in agreement with clinical studies. We argue that, with reasonable assumptions, it is possible to predict (precision ~20%) regrowth delay after radiotherapy and the gain of lifetime due to radiotherapy.
Conclusions: Our oedema-based model provides an early estimation of individual duration of tumour response to radiotherapy and thus, opens the door to the possibility of personalized medicine.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




References
-
- Pallud J, Mandonnet E (2011) Quantitative approach of the natural course of diffuse low‐grade gliomas In: Hayat MA, ed. Tumors of the Central Nervous System, Vol. 2, pp. 163–172. Netherlands: Springer.
-
- Price SJ (2010) Advances in imaging low‐grade gliomas. Adv. Tech. Stand. Neurosurg. 35, 1–34. - PubMed
-
- Pallud J, Fontaine D, Duffau H, Mandonnet E, Sanai N, Taillandier L et al (2010) Natural history of incidental WHO grade II gliomas. Ann. Neurol. 68, 727–733. - PubMed
-
- Daumas‐Duport C, Meder JF, Monsaingeon V, Missir O, Aubin ML, Szikla G (1983) Cerebral gliomas: malignancy, limits and spatial configuration‐comparative data from serial stereotaxic biopsies and computed tomography (a preliminary study based on 50 cases). J. Neuroradiol. 10, 51–80. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

