The significance of interstitial cells in neurogastroenterology
- PMID: 24948131
- PMCID: PMC4102150
- DOI: 10.5056/jnm14060
The significance of interstitial cells in neurogastroenterology
Abstract
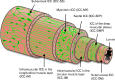
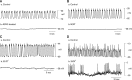
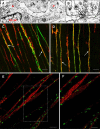
Smooth muscle layers of the gastrointestinal tract consist of a heterogeneous population of cells that include enteric neurons, several classes of interstitial cells of mesenchymal origin, a variety of immune cells and smooth muscle cells (SMCs). Over the last number of years the complexity of the interactions between these cell types has begun to emerge. For example, interstitial cells, consisting of both interstitial cells of Cajal (ICC) and platelet-derived growth factor receptor alpha-positive (PDGFRα(+)) cells generate pacemaker activity throughout the gastrointestinal (GI) tract and also transduce enteric motor nerve signals and mechanosensitivity to adjacent SMCs. ICC and PDGFRα(+) cells are electrically coupled to SMCs possibly via gap junctions forming a multicellular functional syncytium termed the SIP syncytium. Cells that make up the SIP syncytium are highly specialized containing unique receptors, ion channels and intracellular signaling pathways that regulate the excitability of GI muscles. The unique role of these cells in coordinating GI motility is evident by the altered motility patterns in animal models where interstitial cell networks are disrupted. Although considerable advances have been made in recent years on our understanding of the roles of these cells within the SIP syncytium, the full physiological functions of these cells and the consequences of their disruption in GI muscles have not been clearly defined. This review gives a synopsis of the history of interstitial cell discovery and highlights recent advances in structural, molecular expression and functional roles of these cells in the GI tract.
Keywords: Enteric nervous system, Receptor, platelet-derived growth factor alpha, SIP syncytium, Slow waves, TMEM16A protein, mouse.
Figures



References
-
- Cajal SR. Sur les ganglions et plexus nerveux de l’intestin. CR Soc Biol. 1893;45:217–223.
-
- Cajal SR. Nuevas aplicaciones del metodo de coloracion De Golgi. Gaceta Medica Catalana. 1889;12:613–616.
-
- Cajal SR. El plexo de Auerbach de los batracios. Nota sobre el plexo de Auerbach de la rana. Trab Lab Histol Fac Med Barcelona. 1892:23–28.
-
- Cajal SR. Les nouvelles idées sur la structure du système nerveux. Paris: C. Reinwald & Co.; 1894.
-
- Cajal SR. Histologie du systeme nerveux de l’homme et des vertebres. Volume 2. Paris: Maloine; 1911. pp. 891–942.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

