Effects of pump versus twice-daily injection delivery of synthetic parathyroid hormone 1-34 in children with severe congenital hypoparathyroidism
- PMID: 24948345
- PMCID: PMC4174419
- DOI: 10.1016/j.jpeds.2014.04.060
Effects of pump versus twice-daily injection delivery of synthetic parathyroid hormone 1-34 in children with severe congenital hypoparathyroidism
Abstract
Objective: To compare the response with synthetic human parathyroid hormone (PTH) 1-34 delivered by twice-daily injection vs insulin pump in children with severe congenital hypoparathyroidism due to calcium receptor mutation or autoimmune polyglandular syndrome type 1.
Study design: Children and young adults aged 7-20 years with congenital hypoparathyroidism (N = 12) were randomized to receive PTH 1-34, delivered either by twice-daily subcutaneous injection or insulin pump for 13 weeks, followed by crossover to the opposite delivery method. The principal outcome measures were serum and urine calcium levels. Secondary outcomes included serum and urine magnesium and phosphate levels and bone turnover markers.
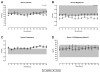
Results: PTH 1-34 delivered via pump produced near normalization of mean serum calcium (2.02 ± 0.05 [pump] vs 1.88 ± 0.03 [injection] mmol/L, P < .05, normal 2.05-2.5 mmol/L), normalized mean urine calcium excretion (5.17 ± 1.10 [pump] vs 6.67 ± 0.76 mmol/24 h/1.73 m(2), P = .3), and significantly reduced markers of bone turnover (P < .02). Serum and urine calcium and magnesium showed a biphasic pattern during twice-daily injection vs minimal fluctuation during pump delivery. The PTH 1-34 dosage was markedly reduced during pump delivery (0.32 ± 0.04 vs 0.85 ± 0.11 μg/kg/d, P < .001), and magnesium supplements were also reduced (P < .001).
Conclusion: Compared with twice-daily delivery, pump delivery of PTH 1-34 provides more physiologic calcium homeostasis and bone turnover in children with severe congenital hypoparathyroidism.
Trial registration: ClinicalTrials.gov NCT00743782.
Published by Mosby, Inc.
Conflict of interest statement
The other authors declare no conflicts of interest.
Figures



References
-
- Rejnmark L, Sikjaer T, Underbjerg L, Mosekilde L. PTH replacement therapy of hypoparathyroidism. Osteoporos Int. 2013;24:1529–36. - PubMed
-
- Weber G, Cazzuffi MA, Frisone F, De Angelis M, Pasolini D, Tomaselli V, et al. Nephrocalcinosis in children and adolescents: sonographic evaluation during long-term treatment with 1,25-dihydrocholecalciferol. Child Nephrol Urol. 1998:273–6. - PubMed
-
- Santos F, Smith MJ, Chan JC. Hypercalciuria associated with long term administration of calcitriol (1,25-dihydroxyvitamin D) Am J Dis Child. 1986;140:139–42. - PubMed
-
- Winer KK, Yanovski JA, Sarani B, Cutler GB., Jr A randomized, crossover trial of once-daily vs twice-daily human parathyroid hormone 1-34 in the treatment of hypoparathyroidism. J Clin Endocrinol Metab. 1998;83:3480–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

