Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition
- PMID: 24948358
- PMCID: PMC4077554
- DOI: 10.1186/1742-2094-11-111
Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition
Abstract
Background: Having the apolipoprotein E4 (APOE-ϵ4) allele is the strongest genetic risk factor for the development of Alzheimer's disease (AD). Accumulation of amyloid beta (Aβ) in the brain is influenced by APOE genotype. Transgenic mice co-expressing five familial AD mutations (5xFAD) in the presence of human APOE alleles (ϵ2, ϵ3 or ϵ4) exhibit APOE genotype-specific differences in early Aβ accumulation, suggesting an interaction between APOE and AD pathology. Whether APOE genotype affects Aβ-plaque-associated neuroinflammation remains unclear. In the current study, we address the role of APOE genotype on Aβ-associated microglial reactivity in the EFAD transgenic mouse model.
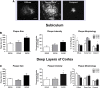
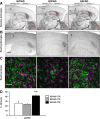
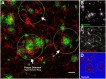
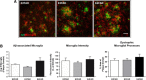
Methods: We analyzed Aβ-induced glial activation in the brains of 6-month-old EFAD transgenic mice (E2FAD, E3FAD and E4FAD). Region-specific morphological profiles of Aβ plaques in EFAD brain sections were compared using immunofluorescence staining. We then determined the degree of glial activation in sites of Aβ deposition while comparing levels of the inflammatory cytokine Interleukin-1β (IL-1β) by ELISA. Finally, we quantified parameters of Aβ-associated microglial reactivity using double-stained EFAD brain sections.
Results: Characterization of Aβ plaques revealed there were larger and more intensely stained plaques in E4FAD mice relative to E2FAD and E3FAD mice. E4FAD mice also had a greater percentage of compact plaques in the subiculum than E3FAD mice. Reactive microglia and dystrophic astrocytes were prominent in EFAD brains, and primarily localized to two sites of significant Aβ deposition: the subiculum and deep layers of the cortex. Cortical levels of IL-1β were nearly twofold greater in E4FAD mice relative to E3FAD mice. To control for differences in levels of Aβ in the different EFAD mice, we analyzed the microglia within domains of specific Aβ deposits. Morphometric analyses revealed increased measures of microglial reactivity in E4FAD mice, including greater dystrophy, increased fluorescence intensity and a higher density of reactive cells surrounding cortical plaques, than in E3FAD mice.
Conclusions: In addition to altering morphological profiles of Aβ deposition, APOE genotype influences Aβ-induced glial activation in the adult EFAD cortex. These data support a role for APOE in modulating Aβ-induced neuroinflammatory responses in AD progression, and support the use of EFAD mice as a suitable model for mechanistic studies of Aβ-associated neuroinflammation.
Figures

References
-
- Selkoe DJ. Resolving controversies on the path to Alzheimer's therapeutics. Nat Med. 2011;17:1060–1065. - PubMed
-
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. discussion 278–284. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

