Role of host GTPases in infection by Listeria monocytogenes
- PMID: 24948362
- PMCID: PMC4656192
- DOI: 10.1111/cmi.12324
Role of host GTPases in infection by Listeria monocytogenes
Abstract
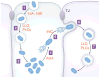
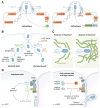
The bacterial pathogen Listeria monocytogenes induces internalization into mammalian cells and uses actin-based motility to spread within tissues. Listeria accomplishes this intracellular life cycle by exploiting or antagonizing several host GTPases. Internalization into human cells is mediated by the bacterial surface proteins InlA or InlB. These two modes of uptake each require a host actin polymerization pathway comprised of the GTPase Rac1, nucleation promotion factors, and the Arp2/3 complex. In addition to Rac1, InlB-mediated internalization involves inhibition of the GTPase Arf6 and participation of Dynamin and septin family GTPases. After uptake, Listeria is encased in host phagosomes. The bacterial protein GAPDH inactivates the human GTPase Rab5, thereby delaying phagosomal acquisition of antimicrobial properties. After bacterial-induced destruction of the phagosome, cytosolic Listeria uses the surface protein ActA to stimulate actin-based motility. The GTPase Dynamin 2 reduces the density of microtubules that would otherwise limit bacterial movement. Cell-to-cell spread results when motile Listeria remodel the host plasma membrane into protrusions that are engulfed by neighbouring cells. The human GTPase Cdc42, its activator Tuba, and its effector N-WASP form a complex with the potential to restrict Listeria protrusions. Bacteria overcome this restriction through two microbial factors that inhibit Cdc42-GTP or Tuba/N-WASP interaction.
© 2014 John Wiley & Sons Ltd.
Figures
References
-
- Alvarez-Dominguez C, Madrazo-Toca F, Fernandez-Prieto L, Vanderkerckhove J, Pareja E, Tobes R, Gomez-Lopez MT, Del Cerro-Vadillo E, Fresno M, fLC, Carrasco-Marin E. Characterization of a Listeria monocytogenes protein interfering with Rab5a. Traffic. 2008;9:325–337. - PubMed
-
- Bershadsky A. Magic touch: how does cell-cells adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. - PubMed
-
- Bierne H, Miki H, Innocenti M, Scita G, Gertler FB, Takenawa T, Cossart P. WASP-related proteins, Abi and Ena/VASP are required for Listeria invasion induced by the Met receptor. J Cell Sci. 2005;118:1537–1547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

