The cortical hem regulates the size and patterning of neocortex
- PMID: 24948604
- PMCID: PMC4197624
- DOI: 10.1242/dev.106914
The cortical hem regulates the size and patterning of neocortex
Abstract
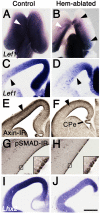
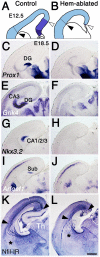
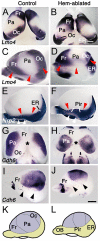
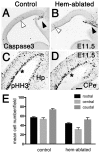
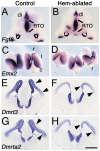
The cortical hem, a source of Wingless-related (WNT) and bone morphogenetic protein (BMP) signaling in the dorsomedial telencephalon, is the embryonic organizer for the hippocampus. Whether the hem is a major regulator of cortical patterning outside the hippocampus has not been investigated. We examined regional organization across the entire cerebral cortex in mice genetically engineered to lack the hem. Indicating that the hem regulates dorsoventral patterning in the cortical hemisphere, the neocortex, particularly dorsomedial neocortex, was reduced in size in late-stage hem-ablated embryos, whereas cortex ventrolateral to the neocortex expanded dorsally. Unexpectedly, hem ablation also perturbed regional patterning along the rostrocaudal axis of neocortex. Rostral neocortical domains identified by characteristic gene expression were expanded, and caudal domains diminished. A similar shift occurs when fibroblast growth factor (FGF) 8 is increased at the rostral telencephalic organizer, yet the FGF8 source was unchanged in hem-ablated brains. Rather we found that hem WNT or BMP signals, or both, have opposite effects to those of FGF8 in regulating transcription factors that control the size and position of neocortical areas. When the hem is ablated a necessary balance is perturbed, and cerebral cortex is rostralized. Our findings reveal a much broader role for the hem in cortical development than previously recognized, and emphasize that two major signaling centers interact antagonistically to pattern cerebral cortex.
Keywords: Embryonic patterning; Fgf8; Mouse; Neocortex; Signaling center; Wnt3a.
© 2014. Published by The Company of Biologists Ltd.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

