Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory
- PMID: 24948795
- PMCID: PMC4061384
- DOI: 10.1523/JNEUROSCI.1385-14.2014
Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory
Abstract
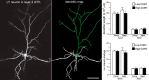
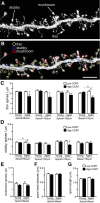
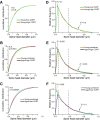
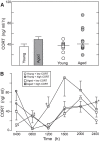
Cognitive decline in aging is marked by considerable variability, with some individuals experiencing significant impairments and others retaining intact functioning. Whereas previous studies have linked elevated hypothalamo-pituitary-adrenal (HPA) axis activity with impaired hippocampal function during aging, the idea has languished regarding whether such differences may underlie the deterioration of other cognitive functions. Here we investigate whether endogenous differences in HPA activity are predictive of age-related impairments in prefrontal structural and behavioral plasticity. Young and aged rats (4 and 21 months, respectively) were partitioned into low or high HPA activity, based upon averaged values of corticosterone release from each animal obtained from repeated sampling across a 24 h period. Pyramidal neurons in the prelimbic area of medial prefrontal cortex were selected for intracellular dye filling, followed by 3D imaging and analysis of dendritic spine morphometry. Aged animals displayed dendritic spine loss and altered geometric characteristics; however, these decrements were largely accounted for by the subgroup bearing elevated corticosterone. Moreover, high adrenocortical activity in aging was associated with downward shifts in frequency distributions for spine head diameter and length, whereas aged animals with low corticosterone showed an upward shift in these indices. Follow-up behavioral experiments revealed that age-related spatial working memory deficits were exacerbated by increased HPA activity. By contrast, variations in HPA activity in young animals failed to impact structural or behavioral plasticity. These data implicate the cumulative exposure to glucocorticoids as a central underlying process in age-related prefrontal impairment and define synaptic features accounting for different trajectories in age-related cognitive function.
Keywords: HPA axis; corticosterone; medial prefrontal cortex; prelimbic; working memory.
Copyright © 2014 the authors 0270-6474/14/348387-11$15.00/0.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
