Coordinated within-trial dynamics of low-frequency neural rhythms controls evidence accumulation
- PMID: 24948807
- PMCID: PMC6608218
- DOI: 10.1523/JNEUROSCI.3801-13.2014
Coordinated within-trial dynamics of low-frequency neural rhythms controls evidence accumulation
Abstract
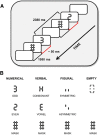
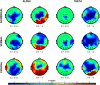
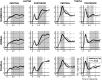
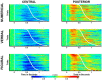
Higher cognitive functions, such as human perceptual decision making, require information processing and transmission across wide-spread cortical networks. Temporally synchronized neural firing patterns are advantageous for efficiently representing and transmitting information within and between assemblies. Computational, empirical, and conceptual considerations all lead to the expectation that the informational redundancy of neural firing rates is positively related to their synchronization. Recent theorizing and initial evidence also suggest that the coding of stimulus characteristics and their integration with behavioral goal states require neural interactions across a hierarchy of timescales. However, most studies thus have focused on neural activity in a single frequency range or on a restricted set of brain regions. Here we provide evidence for cooperative spatiotemporal dynamics of slow and fast EEG signals during perceptual decision making at the single-trial level. Participants performed three masked two-choice decision tasks, one each with numerical, verbal, or figural content. Decrements in posterior α power (8-14 Hz) were paralleled by increments in high-frequency (>30 Hz) signal entropy in trials demanding active sensory processing. Simultaneously, frontocentral θ power (4-7 Hz) increased, indicating evidence integration. The coordinated α/θ dynamics were tightly linked to decision speed and remarkably similar across tasks, suggesting a domain-general mechanism. In sum, we demonstrate an inverse association between decision-related changes in widespread low-frequency power and local high-frequency entropy. The cooperation among mechanisms captured by these changes enhances the informational density of neural response patterns and qualifies as a neural coding system in the service of perceptual decision making.
Keywords: EEG; decision making; entropy; neural oscillations; single-trial analyses; synchronization.
Copyright © 2014 the authors 0270-6474/14/348519-10$15.00/0.
Figures




Comment in
-
The temporal dynamics of evidence accumulation in the brain.J Neurosci. 2014 Oct 15;34(42):13870-1. doi: 10.1523/JNEUROSCI.3251-14.2014. J Neurosci. 2014. PMID: 25319683 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
