Neurodegeneration by activation of the microglial complement-phagosome pathway
- PMID: 24948809
- PMCID: PMC6608212
- DOI: 10.1523/JNEUROSCI.5002-13.2014
Neurodegeneration by activation of the microglial complement-phagosome pathway
Abstract
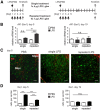
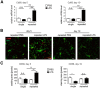
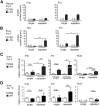
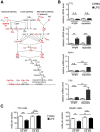
Systemic inflammatory reactions have been postulated to exacerbate neurodegenerative diseases via microglial activation. We now demonstrate in vivo that repeated systemic challenge of mice over four consecutive days with bacterial LPS maintained an elevated microglial inflammatory phenotype and induced loss of dopaminergic neurons in the substantia nigra. The same total cumulative LPS dose given within a single application did not induce neurodegeneration. Whole-genome transcriptome analysis of the brain demonstrated that repeated systemic LPS application induced an activation pattern involving the classical complement system and its associated phagosome pathway. Loss of dopaminergic neurons induced by repeated systemic LPS application was rescued in complement C3-deficient mice, confirming the involvement of the complement system in neurodegeneration. Our data demonstrate that a phagosomal inflammatory response of microglia is leading to complement-mediated loss of dopaminergic neurons.
Keywords: complement; microglia; neurodegeneration; neuroinflammation; phagosome; transcriptome.
Copyright © 2014 the authors 0270-6474/14/348546-11$15.00/0.
Figures



References
-
- Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience. 2009;161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. - DOI - PMC - PubMed
-
- Benjamini Y, Hochberg T. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
