Quantitative secretome and glycome of primary human adipocytes during insulin resistance
- PMID: 24948903
- PMCID: PMC4055909
- DOI: 10.1186/1559-0275-11-20
Quantitative secretome and glycome of primary human adipocytes during insulin resistance
Abstract
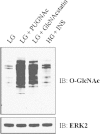
Adipose tissue is both an energy storage depot and an endocrine organ. The impaired regulation of the secreted proteins of adipose tissue, known as adipocytokines, observed during obesity contributes to the onset of whole-body insulin resistance and the pathobiology of type 2 diabetes mellitus (T2DM). In addition, the global elevation of the intracellular glycosylation of proteins by O-linked β-N-acetylglucosamine (O-GlcNAc) via either genetic or pharmacological methods is sufficient to induce insulin resistance in both cultured cells and animal models. The elevation of global O-GlcNAc levels is associated with the altered expression of many adipocytokines. We have previously characterized the rodent adipocyte secretome during insulin sensitive and insulin resistant conditions. Here, we characterize and quantify the secretome and glycome of primary human adipocytes during insulin responsive and insulin resistant conditions generated by the classical method of hyperglycemia and hyperinsulinemia or by the pharmacological manipulation of O-GlcNAc levels. Using a proteomic approach, we identify 190 secreted proteins and report a total of 20 up-regulated and 6 down-regulated proteins that are detected in both insulin resistant conditions. Moreover, we apply glycomic techniques to examine (1) the sites of N-glycosylation on secreted proteins, (2) the structures of complex N- and O-glycans, and (3) the relative abundance of complex N- and O-glycans structures in insulin responsive and insulin resistant conditions. We identify 91 N-glycosylation sites derived from 51 secreted proteins, as well as 155 and 29 released N- and O-glycans respectively. We go on to quantify many of the N- and O-glycan structures between insulin responsive and insulin resistance conditions demonstrating no significant changes in complex glycosylation in the time frame for the induction of insulin resistance. Thus, our data support that the O-GlcNAc modification is involved in the regulation of adipocytokine secretion upon the induction of insulin resistance in human adipocytes.
Keywords: Adipocytokine; Glycomics; Insulin resistance; N-linked; O-GlcNAc; O-linked; Shotgun proteomics; Tandem mass spectrometry; Type 2 diabetes.
Figures



References
-
- Prevention., C. f. D. C. a. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: US: Department of Health and Human Services, Centers for Disease Control and Prevention; 2011.
-
- Harwood HJ. , JrThe adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2011. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
