Complement yourself: Transcomplementation rescues partially folded mutant proteins
- PMID: 24949105
- PMCID: PMC4059760
- DOI: 10.1007/s12551-014-0137-3
Complement yourself: Transcomplementation rescues partially folded mutant proteins
Abstract
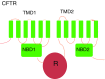
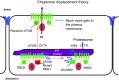
Cystic Fibrosis (CF) is an autosomal disease associated with malfunction in fluid and electrolyte transport across several mucosal membranes. The most common mutation in CF is an in-frame three-base pair deletion that removes a phenylalanine at position 508 in the first nucleotide-binding domain of the cystic fibrosis conductance regulator (CFTR) chloride channel. This mutation has been studied extensively and leads to biosynthetic arrest of the protein in the endoplasmic reticulum and severely reduced channel activity. This review discusses a novel method of rescuing ΔF508 with transcomplementation, which occurs when smaller fragments of CFTR containing the wild-type nucleotide binding domain are co-expressed with the ΔF508 deletion mutant. Transcomplementation rescues the processing and channel activity of ΔF508 and reduces its rate of degradation in airway epithelial cells. To apply transcomplementation as a therapy would require that the cDNA encoding the truncated CFTR be delivered to cells. We also discuss a gene therapeutic approach based on delivery of a truncated form of CFTR to airway cells using adeno-associated viral vectors.
Figures



Similar articles
-
Rescuing cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by transcomplementation.Proc Natl Acad Sci U S A. 2004 May 25;101(21):8221-6. doi: 10.1073/pnas.0400459101. Epub 2004 May 12. Proc Natl Acad Sci U S A. 2004. PMID: 15141088 Free PMC article.
-
A truncated CFTR protein rescues endogenous DeltaF508-CFTR and corrects chloride transport in mice.FASEB J. 2009 Nov;23(11):3743-51. doi: 10.1096/fj.08-127878. Epub 2009 Jul 20. FASEB J. 2009. PMID: 19620404 Free PMC article.
-
Transcomplementation by a truncation mutant of cystic fibrosis transmembrane conductance regulator (CFTR) enhances ΔF508 processing through a biomolecular interaction.J Biol Chem. 2013 Apr 12;288(15):10505-12. doi: 10.1074/jbc.M112.420489. Epub 2013 Mar 5. J Biol Chem. 2013. PMID: 23463513 Free PMC article.
-
Can correcting the ΔF508-CFTR proteostasis-defect rescue CF lung disease?Curr Mol Med. 2012 Aug;12(7):860-71. doi: 10.2174/156652412801318773. Curr Mol Med. 2012. PMID: 22697346 Review.
-
Pharmacological treatment of the ion transport defect in cystic fibrosis.Expert Opin Investig Drugs. 2001 Jan;10(1):1-19. doi: 10.1517/13543784.10.1.1. Expert Opin Investig Drugs. 2001. PMID: 11116277 Review.
Cited by
-
Adeno-Associated Virus (AAV) gene therapy for cystic fibrosis: current barriers and recent developments.Expert Opin Biol Ther. 2017 Oct;17(10):1265-1273. doi: 10.1080/14712598.2017.1347630. Epub 2017 Jul 6. Expert Opin Biol Ther. 2017. PMID: 28657358 Free PMC article. Review.
-
Amelioration of airway and GI disease in G551D-CF ferrets by AAV1 and AAV6.Gene Ther. 2024 Sep;31(9-10):499-510. doi: 10.1038/s41434-024-00469-7. Epub 2024 Jul 28. Gene Ther. 2024. PMID: 39069560
-
Short-Term Steroid Treatment of Rhesus Macaque Increases Transduction.Hum Gene Ther. 2022 Feb;33(3-4):131-147. doi: 10.1089/hum.2021.239. Epub 2022 Jan 7. Hum Gene Ther. 2022. PMID: 34806411 Free PMC article.
-
AAV1-CFTR preferentially transduces cysts and reduces cyst size in a mouse model of ADPKD.Am J Physiol Cell Physiol. 2025 Jun 1;328(6):C1783-C1792. doi: 10.1152/ajpcell.00057.2025. Epub 2025 Apr 16. Am J Physiol Cell Physiol. 2025. PMID: 40237039 Free PMC article.
-
Gene therapy for cystic fibrosis: Challenges and prospects.Front Pharmacol. 2022 Oct 11;13:1015926. doi: 10.3389/fphar.2022.1015926. eCollection 2022. Front Pharmacol. 2022. PMID: 36304167 Free PMC article. Review.
References
-
- Ahner A, Gong X, Schmidt BZ, Peters KW, Rabeh WM, Thibodeau PH, Lukacs GL, Frizzell RA. Small heat shock proteins target mutant cystic fibrosis transmembrane conductance regulator for degradation via a small ubiquitin-like modifier-dependent pathway. Mol Biol Cell. 2013;24:74–84. doi: 10.1091/mbc.E12-09-0678. - DOI - PMC - PubMed
-
- Atwell S, Brouillette CG, Conners K, Emtage S, Gheyi T, Guggino WB, Hendle J, Hunt JF, Lewis HA, Lu F, Protasevich II, Rodgers LA, Romero R, Wasserman SR, Weber PC, Wetmore D, Zhang FF, Zhao X. Structures of a minimal human CFTR first nucleotide-binding domain as a monomer, head-to-tail homodimer, and pathogenic mutant. Protein Eng Des Sel. 2010;23:375–384. doi: 10.1093/protein/gzq004. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

