Complement yourself: Transcomplementation rescues partially folded mutant proteins
- PMID: 24949105
- PMCID: PMC4059760
- DOI: 10.1007/s12551-014-0137-3
Complement yourself: Transcomplementation rescues partially folded mutant proteins
Abstract
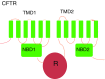
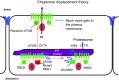
Cystic Fibrosis (CF) is an autosomal disease associated with malfunction in fluid and electrolyte transport across several mucosal membranes. The most common mutation in CF is an in-frame three-base pair deletion that removes a phenylalanine at position 508 in the first nucleotide-binding domain of the cystic fibrosis conductance regulator (CFTR) chloride channel. This mutation has been studied extensively and leads to biosynthetic arrest of the protein in the endoplasmic reticulum and severely reduced channel activity. This review discusses a novel method of rescuing ΔF508 with transcomplementation, which occurs when smaller fragments of CFTR containing the wild-type nucleotide binding domain are co-expressed with the ΔF508 deletion mutant. Transcomplementation rescues the processing and channel activity of ΔF508 and reduces its rate of degradation in airway epithelial cells. To apply transcomplementation as a therapy would require that the cDNA encoding the truncated CFTR be delivered to cells. We also discuss a gene therapeutic approach based on delivery of a truncated form of CFTR to airway cells using adeno-associated viral vectors.
Figures



References
-
- Ahner A, Gong X, Schmidt BZ, Peters KW, Rabeh WM, Thibodeau PH, Lukacs GL, Frizzell RA. Small heat shock proteins target mutant cystic fibrosis transmembrane conductance regulator for degradation via a small ubiquitin-like modifier-dependent pathway. Mol Biol Cell. 2013;24:74–84. doi: 10.1091/mbc.E12-09-0678. - DOI - PMC - PubMed
-
- Atwell S, Brouillette CG, Conners K, Emtage S, Gheyi T, Guggino WB, Hendle J, Hunt JF, Lewis HA, Lu F, Protasevich II, Rodgers LA, Romero R, Wasserman SR, Weber PC, Wetmore D, Zhang FF, Zhao X. Structures of a minimal human CFTR first nucleotide-binding domain as a monomer, head-to-tail homodimer, and pathogenic mutant. Protein Eng Des Sel. 2010;23:375–384. doi: 10.1093/protein/gzq004. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

