Managing the potential and pitfalls during clinical translation of emerging stem cell therapies
- PMID: 24949190
- PMCID: PMC4049443
- DOI: 10.1186/2001-1326-3-10
Managing the potential and pitfalls during clinical translation of emerging stem cell therapies
Abstract
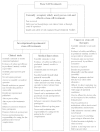
We are moving into a new era of stem cell research where many possibilities for treatment of degenerative, chronic and/or fatal diseases and injuries are becoming primed for clinical trial. These reports have led millions of people worldwide to hope that regenerative medicine is about to revolutionise biomedicine: either through transplantation of cells grown in the laboratory, or by finding ways to stimulate a patient's intrinsic stem cells to repair diseased and damaged organs. While major contributions of stem cells to drug discovery, safety and efficacy testing, as well as modelling 'diseases in a dish' are also expected, it is the in vivo use of stem cells that has captured the general public's attention. However, public misconceptions of stem cell potential and applications can leave patients vulnerable to the influences of profit driven entities selling unproven treatments without solid scientific basis or appropriate clinical testing or follow up. This review provides a brief history of stem cell clinical translation together with an overview of the properties, potential, and current clinical application of various stem cell types. In doing so it presents a clearer picture of the inherent risks and opportunities associated with stem cell research translation, and thus offers a framework to help realise invested expectations more quickly, safely and effectively.
Keywords: Allogeneic; Autologous; Clinical trial; Homologous; Pluripotent stem cell; Regenerative medicine; Regulations; Tissue specific stem cell; Unproven treatment.
Figures
References
-
- Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;3:1366–1369. - PubMed
-
- Singhal S, Powles R, Kulkarni S, Treleaven J, Sirohi B, Millar B, Shepherd V, Saso R, Rowland A, Long S, Cabral S, Horton C, Mehta J. Comparison of marrow and blood cell yields from the same donors in a double-blind, randomized study of allogeneic marrow vs blood stem cell transplantation. Bone Marrow Transplant. 2000;3:501–505. doi: 10.1038/sj.bmt.1702173. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources