Circadian modulation of the Cl(-) equilibrium potential in the rat suprachiasmatic nuclei
- PMID: 24949446
- PMCID: PMC4052495
- DOI: 10.1155/2014/424982
Circadian modulation of the Cl(-) equilibrium potential in the rat suprachiasmatic nuclei
Abstract
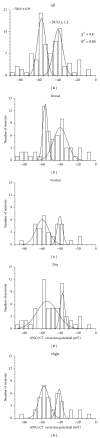
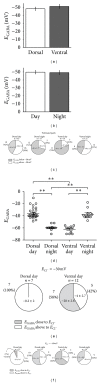
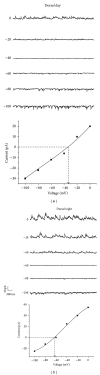
The suprachiasmatic nuclei (SCN) constitute a circadian clock in mammals, where γ-amino-butyric acid (GABA) neurotransmission prevails and participates in different aspects of circadian regulation. Evidence suggests that GABA has an excitatory function in the SCN in addition to its typical inhibitory role. To examine this possibility further, we determined the equilibrium potential of GABAergic postsynaptic currents (E(GABA)) at different times of the day and in different regions of the SCN, using either perforated or whole cell patch clamp. Our results indicate that during the day most neurons in the dorsal SCN have an E(GABA) close to -30 mV while in the ventral SCN they have an E(GABA) close to -60 mV; this difference reverses during the night, in the dorsal SCN neurons have an E(GABA) of -60 mV and in the ventral SCN they have an E(GABA) of -30 mV. The depolarized equilibrium potential can be attributed to the activity of the Na(+)-K(+)-2Cl(-) (NKCC) cotransporter since the equilibrium potential becomes more negative following addition of the NKCC blocker bumetanide. Our results suggest an excitatory role for GABA in the SCN and further indicate both time (day versus night) and regional (dorsal versus ventral) modulation of E(GABA) in the SCN.
Figures






References
-
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York, NY, USA: Oxford University Press; 1991.
-
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Research. 2001;916(1-2):172–191. - PubMed
-
- Morin LP, Shivers K-Y, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137(4):1285–1297. - PubMed
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

