Gremlin activates the Smad pathway linked to epithelial mesenchymal transdifferentiation in cultured tubular epithelial cells
- PMID: 24949470
- PMCID: PMC4052161
- DOI: 10.1155/2014/802841
Gremlin activates the Smad pathway linked to epithelial mesenchymal transdifferentiation in cultured tubular epithelial cells
Abstract
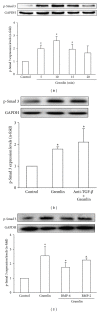
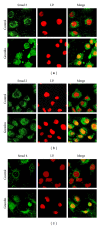
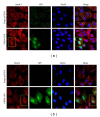
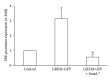
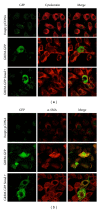
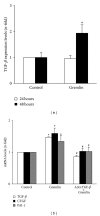
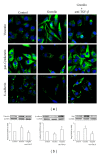
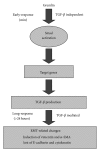
Gremlin is a developmental gene upregulated in human chronic kidney disease and in renal cells in response to transforming growth factor-β (TGF-β). Epithelial mesenchymal transition (EMT) is one process involved in renal fibrosis. In tubular epithelial cells we have recently described that Gremlin induces EMT and acts as a downstream TGF-β mediator. Our aim was to investigate whether Gremlin participates in EMT by the regulation of the Smad pathway. Stimulation of human tubular epithelial cells (HK2) with Gremlin caused an early activation of the Smad signaling pathway (Smad 2/3 phosphorylation, nuclear translocation, and Smad-dependent gene transcription). The blockade of TGF-β, by a neutralizing antibody against active TGF-β, did not modify Gremlin-induced early Smad activation. These data show that Gremlin directly, by a TGF-β independent process, activates the Smad pathway. In tubular epithelial cells long-term incubation with Gremlin increased TGF-β production and caused a sustained Smad activation and a phenotype conversion into myofibroblasts-like cells. Smad 7 overexpression, which blocks Smad 2/3 activation, diminished EMT changes observed in Gremlin-transfected tubuloepithelial cells. TGF-β neutralization also diminished Gremlin-induced EMT changes. In conclusion, we propose that Gremlin could participate in renal fibrosis by inducing EMT in tubular epithelial cells through activation of Smad pathway and induction of TGF-β.
Figures
Similar articles
-
Gremlin is a downstream profibrotic mediator of transforming growth factor-beta in cultured renal cells.Nephron Exp Nephrol. 2012;122(1-2):62-74. doi: 10.1159/000346575. Epub 2013 Mar 14. Nephron Exp Nephrol. 2012. PMID: 23548835
-
TGF-β2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF.Biochem Biophys Res Commun. 2014 May 16;447(4):689-95. doi: 10.1016/j.bbrc.2014.04.068. Epub 2014 Apr 19. Biochem Biophys Res Commun. 2014. PMID: 24755068
-
Curcumin inhibits transforming growth factor-β1-induced EMT via PPARγ pathway, not Smad pathway in renal tubular epithelial cells.PLoS One. 2013;8(3):e58848. doi: 10.1371/journal.pone.0058848. Epub 2013 Mar 27. PLoS One. 2013. PMID: 23544048 Free PMC article.
-
[Aberrant Activation Mechanism of TGF-β Signaling in Epithelial-mesenchymal Transition].Yakugaku Zasshi. 2021;141(11):1229-1234. doi: 10.1248/yakushi.21-00143. Yakugaku Zasshi. 2021. PMID: 34719542 Review. Japanese.
-
The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy.Cytokine Growth Factor Rev. 2011 Jun;22(3):131-9. doi: 10.1016/j.cytogfr.2011.06.002. Epub 2011 Jul 14. Cytokine Growth Factor Rev. 2011. PMID: 21757394 Review.
Cited by
-
GREM1/PPP2R3A expression in heterogeneous fibroblasts initiates pulmonary fibrosis.Cell Biosci. 2022 Aug 6;12(1):123. doi: 10.1186/s13578-022-00860-0. Cell Biosci. 2022. PMID: 35933397 Free PMC article.
-
The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation.J Biol Chem. 2022 Feb;298(2):101530. doi: 10.1016/j.jbc.2021.101530. Epub 2021 Dec 23. J Biol Chem. 2022. PMID: 34953859 Free PMC article. Review.
-
Gremlin: a complex molecule regulating wound healing and fibrosis.Cell Mol Life Sci. 2021 Dec;78(24):7917-7923. doi: 10.1007/s00018-021-03964-x. Epub 2021 Nov 3. Cell Mol Life Sci. 2021. PMID: 34731251 Free PMC article. Review.
-
Acute Kidney Injury and Progression of Diabetic Kidney Disease.Adv Chronic Kidney Dis. 2018 Mar;25(2):166-180. doi: 10.1053/j.ackd.2017.12.005. Adv Chronic Kidney Dis. 2018. PMID: 29580581 Free PMC article. Review.
-
Saikosaponin D Inhibits Peritoneal Fibrosis in Rats With Renal Failure by Regulation of TGFβ1/ BMP7 / Gremlin1/ Smad Pathway.Front Pharmacol. 2021 Oct 1;12:628671. doi: 10.3389/fphar.2021.628671. eCollection 2021. Front Pharmacol. 2021. PMID: 34721005 Free PMC article.
References
-
- Roxburgh SA, Murphy M, Pollock CA, Brazil DP. Recapitulation of embryological programmes in renal fibrosis—the importance of epithelial cell plasticity and developmental genes. Nephron Physiology. 2006;103(3):p139–p148. - PubMed
-
- McMahon R, Murphy M, Clarkson M, et al. IHG-2, a mesangial cell gene induced by high glucose, is human gremlin: regulation by extracellular glucose concentration, cyclic mechanical strain, and transforming growth factor-β1. The Journal of Biological Chemistry. 2000;275(14):9901–9904. - PubMed
-
- Topol LZ, Bardot B, Zhang Q, et al. Biosynthesis, post-translation modification, and functional characterization of Drm/Gremlin. The Journal of Biological Chemistry. 2000;275(12):8785–8793. - PubMed
-
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131(14):3401–3410. - PubMed
-
- Dolan V, Murphy M, Sadlier D, et al. Expression of gremlin, a bone morphogenetic protein antagonist, in human diabetic nephropathy. American Journal of Kidney Diseases. 2005;45(6):1034–1039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

