The evolution of nonsteroidal antiestrogens to become selective estrogen receptor modulators
- PMID: 24949934
- PMCID: PMC4192084
- DOI: 10.1016/j.steroids.2014.06.009
The evolution of nonsteroidal antiestrogens to become selective estrogen receptor modulators
Abstract
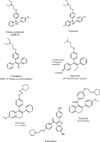
The discovery of the first nonsteroidal antiestrogen ethamoxytriphetol (MER25) in 1958, opened the door to a wide range of clinical applications. However, the finding that ethamoxytriphetol was a "morning after" pill in laboratory animals, energized the pharmaceutical industry to discover more potent derivatives. In the wake of the enormous impact of the introduction of the oral contraceptive worldwide, contraceptive research was a central focus in the early 1960's. Numerous compounds were discovered e.g., clomiphene, nafoxidine, and tamoxifen, but the fact that clinical studies showed no contraceptive actions, but, in fact, induced ovulation, dampened enthusiasm for clinical development. Only clomiphene moved forward to pioneer an application to induce ovulation in subfertile women. The fact that all the compounds were antiestrogenic made an application in patients to treat estrogen responsive breast cancer, an obvious choice. However, toxicities and poor projected commercial returns severely retarded clinical development for two decades. In the 1970's a paradigm shift in the laboratory to advocate long term adjuvant tamoxifen treatment for early (non-metastatic) breast cancer changed medical care and dramatically increased survivorship. Tamoxifen pioneered that paradigm shift but it became the medicine of choice in a second paradigm shift for preventing breast cancer during the 1980's and 1990's. This was not surprising as it was the only medicine available and there was laboratory and clinical evidence for the eventual success of this application. Tamoxifen is the first medicine to be approved by the Food and Drug Administration (FDA) to reduce the risk of breast cancer in women at high risk. But it was the re-evaluation of the toxicology of tamoxifen in the 1980's and the finding that there was both carcinogenic potential and a significant, but small, risk of endometrial cancer in postmenopausal women that led to a third paradigm shift to identify applications for selective estrogen receptor (ER) modulation. This idea was to establish a new group of medicines now called selective ER modulators (SERMs). Today there are 5 SERMs FDA approved (one other in Europe) for applications ranging from the reduction of breast cancer risk and osteoporosis to the reduction of menopausal hot flashes and improvements in dyspareunia and vaginal lubrication. This article charts the origins of the current path for progress in women's health with SERMs.
Keywords: Breast cancer; Endometrial cancer; Osteoporosis; Women’s health.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
-
- Lerner LJ, Holthaus FJ, Jr, Thompson CR. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958 Sep;63(3):295–318. - PubMed
-
- Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. Preliminary report. JAMA. 1961 Oct 14;178:101–104. - PubMed
-
- Lerner LJ. The first nonsteroidal antioestrogen - MER25. In: Sutherland RL, Jordan VC, editors. Non-Steroidal Antioestrogens: Molecular Pharmacology and Antitumour Activity. Sydney and New York: Academic Press; 1981. pp. 1–16.
-
- Avigan J, Steinberg D, Vroman HE, Thompson MJ, Mosettig E. Studies of cholesterol biosynthesis. I. The identification of desmosterol in serum and tissues of animals and man treated with MER-29. J Biol Chem. 1960 Nov;235:3123–3126. - PubMed
-
- Laughlin RC, Carey TF. Cataracts in patients treated with triparanol. JAMA. 1962 Jul 28;181:339–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

