Multiple tissue-specific requirements for the BMP antagonist Noggin in development of the mammalian craniofacial skeleton
- PMID: 24949938
- PMCID: PMC4399715
- DOI: 10.1016/j.ydbio.2014.06.006
Multiple tissue-specific requirements for the BMP antagonist Noggin in development of the mammalian craniofacial skeleton
Abstract
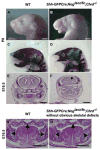
Proper morphogenesis is essential for both form and function of the mammalian craniofacial skeleton, which consists of more than twenty small cartilages and bones. Skeletal elements that support the oral cavity are derived from cranial neural crest cells (NCCs) that develop in the maxillary and mandibular buds of pharyngeal arch 1 (PA1). Bone Morphogenetic Protein (BMP) signaling has been implicated in most aspects of craniofacial skeletogenesis, including PA1 development. However, the roles of the BMP antagonist Noggin in formation of the craniofacial skeleton remain unclear, in part because of its multiple domains of expression during formative stages. Here we used a tissue-specific gene ablation approach to assess roles of Noggin (Nog) in two different tissue domains potentially relevant to mandibular and maxillary development. We found that the axial midline domain of Nog expression is critical to promote PA1 development in early stages, necessary for adequate outgrowth of the mandibular bud. Subsequently, Nog expression in NCCs regulates craniofacial cartilage and bone formation. Mice lacking Nog in NCCs have an enlarged mandible that results from increased cell proliferation in and around Meckel׳s cartilage. These mutants also show complete secondary cleft palate, most likely due to inhibition of posterior palatal shelf elevation by disrupted morphology of the developing skull base. Our findings demonstrate multiple roles of Noggin in different domains for craniofacial skeletogenesis, and suggest an indirect mechanism for secondary cleft palate in Nog mutants that may be relevant to human cleft palate as well.
Keywords: Bone morphogenetic protein; Mandible; Mouse development; Neural crest; Noggin; Palate.
Copyright © 2014. Published by Elsevier Inc.
Figures







Similar articles
-
Endogenous bone morphogenetic protein antagonists regulate mammalian neural crest generation and survival.Dev Dyn. 2006 Sep;235(9):2507-20. doi: 10.1002/dvdy.20891. Dev Dyn. 2006. PMID: 16894609 Free PMC article.
-
The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth.Dev Biol. 2001 Dec 15;240(2):457-73. doi: 10.1006/dbio.2001.0479. Dev Biol. 2001. PMID: 11784076
-
Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis.Dev Biol. 2010 Nov 1;347(1):109-21. doi: 10.1016/j.ydbio.2010.08.014. Epub 2010 Aug 19. Dev Biol. 2010. PMID: 20727875 Free PMC article.
-
BMP signalling in craniofacial development.Int J Dev Biol. 2006;50(6):511-21. doi: 10.1387/ijdb.052101xn. Int J Dev Biol. 2006. PMID: 16741866 Review.
-
Interplay between bone morphogenetic proteins and cognate binding proteins in bone and cartilage development: noggin, chordin and DAN.Arthritis Res. 2001;3(1):1-5. doi: 10.1186/ar133. Epub 2000 Nov 14. Arthritis Res. 2001. PMID: 11178121 Free PMC article. Review.
Cited by
-
Wnt signaling in orofacial clefts: crosstalk, pathogenesis and models.Dis Model Mech. 2019 Feb 4;12(2):dmm037051. doi: 10.1242/dmm.037051. Dis Model Mech. 2019. PMID: 30760477 Free PMC article. Review.
-
Common mechanisms in development and disease: BMP signaling in craniofacial development.Cytokine Growth Factor Rev. 2016 Feb;27:129-39. doi: 10.1016/j.cytogfr.2015.11.004. Epub 2015 Nov 24. Cytokine Growth Factor Rev. 2016. PMID: 26747371 Free PMC article. Review.
-
Why Does the Face Predict the Brain? Neural Crest Induction, Craniofacial Morphogenesis, and Neural Circuit Development.Front Physiol. 2020 Dec 11;11:610970. doi: 10.3389/fphys.2020.610970. eCollection 2020. Front Physiol. 2020. PMID: 33362582 Free PMC article. Review.
-
Regulatory mechanisms of jaw bone and tooth development.Curr Top Dev Biol. 2019;133:91-118. doi: 10.1016/bs.ctdb.2018.12.013. Epub 2019 Feb 11. Curr Top Dev Biol. 2019. PMID: 30902260 Free PMC article. Review.
-
PLGA-based control release of Noggin blocks the premature fusion of cranial sutures caused by retinoic acid.Appl Microbiol Biotechnol. 2019 Jan;103(1):291-301. doi: 10.1007/s00253-018-9457-8. Epub 2018 Nov 3. Appl Microbiol Biotechnol. 2019. PMID: 30392121 Free PMC article.
References
-
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. - PubMed
-
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. - PubMed
-
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

