Natural neural projection dynamics underlying social behavior
- PMID: 24949967
- PMCID: PMC4123133
- DOI: 10.1016/j.cell.2014.05.017
Natural neural projection dynamics underlying social behavior
Abstract
Social interaction is a complex behavior essential for many species and is impaired in major neuropsychiatric disorders. Pharmacological studies have implicated certain neurotransmitter systems in social behavior, but circuit-level understanding of endogenous neural activity during social interaction is lacking. We therefore developed and applied a new methodology, termed fiber photometry, to optically record natural neural activity in genetically and connectivity-defined projections to elucidate the real-time role of specified pathways in mammalian behavior. Fiber photometry revealed that activity dynamics of a ventral tegmental area (VTA)-to-nucleus accumbens (NAc) projection could encode and predict key features of social, but not novel object, interaction. Consistent with this observation, optogenetic control of cells specifically contributing to this projection was sufficient to modulate social behavior, which was mediated by type 1 dopamine receptor signaling downstream in the NAc. Direct observation of deep projection-specific activity in this way captures a fundamental and previously inaccessible dimension of mammalian circuit dynamics.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


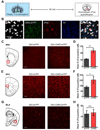
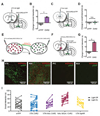
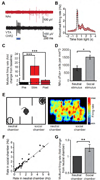
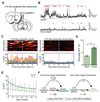

References
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. - PubMed
-
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature neuroscience. 2006;9:133–139. - PubMed
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. Journal of neural engineering. 2007;4:S143–S156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

