Conservation of Ca2+/calmodulin regulation across Na and Ca2+ channels
- PMID: 24949975
- PMCID: PMC4349408
- DOI: 10.1016/j.cell.2014.04.035
Conservation of Ca2+/calmodulin regulation across Na and Ca2+ channels
Abstract
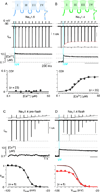
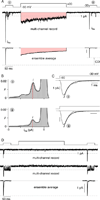
Voltage-gated Na and Ca2+ channels comprise distinct ion channel superfamilies, yet the carboxy tails of these channels exhibit high homology, hinting at a long-shared and purposeful module. For different Ca2+ channels, carboxyl-tail interactions with calmodulin do elaborate robust and similar forms of Ca2+ regulation. However, Na channels have only shown subtler Ca2+ modulation that differs among reports, challenging attempts at unified understanding. Here, by rapid Ca2+ photorelease onto Na channels, we reset this view of Na channel regulation. For cardiac-muscle channels (NaV1.5), reported effects from which most mechanistic proposals derive, we observe no Ca2+ modulation. Conversely, for skeletal-muscle channels (NaV1.4), we uncover fast Ca2+ regulation eerily similar to that of Ca2+ channels. Channelopathic myotonia mutations halve NaV1.4 Ca2+ regulation, and transplanting the NaV1.4 carboxy tail onto Ca2+ channels recapitulates Ca2+ regulation. Thus, we argue for the persistence and physiological relevance of an ancient Ca2+ regulatory module across Na and Ca2+ channels.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

