Pleiotropic effect of AccD5 and AccE5 depletion in acyl-coenzyme A carboxylase activity and in lipid biosynthesis in mycobacteria
- PMID: 24950047
- PMCID: PMC4064979
- DOI: 10.1371/journal.pone.0099853
Pleiotropic effect of AccD5 and AccE5 depletion in acyl-coenzyme A carboxylase activity and in lipid biosynthesis in mycobacteria
Erratum in
-
Correction: Pleiotropic Effect of AccD5 and AccE5 Depletion in Acyl-Coenzyme A Carboxylase Activity and in Lipid Biosynthesis in Mycobacteria.PLoS One. 2020 Nov 11;15(11):e0242528. doi: 10.1371/journal.pone.0242528. eCollection 2020. PLoS One. 2020. PMID: 33175908 Free PMC article.
Abstract
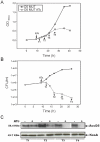
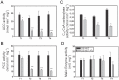
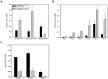
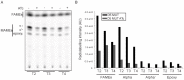
Mycobacteria contain a large variety of fatty acids which are used for the biosynthesis of several complex cell wall lipids that have been implicated in the ability of the organism to resist host defenses. The building blocks for the biosynthesis of all these lipids are provided by a fairly complex set of acyl-CoA carboxylases (ACCases) whose subunit composition and roles within these organisms have not yet been clearly established. Previous biochemical and structural studies provided strong evidences that ACCase 5 from Mycobacterium tuberculosis is formed by the AccA3, AccD5 and AccE5 subunits and that this enzyme complex carboxylates acetyl-CoA and propionyl-CoA with a clear substrate preference for the latest. In this work we used a genetic approach to unambiguously demonstrate that the products of both accD5 and accE5 genes are essential for the viability of Mycobacterium smegmatis. By obtaining a conditional mutant on the accD5-accE5 operon, we also demonstrated that the main physiological role of this enzyme complex was to provide the substrates for fatty acid and mycolic acid biosynthesis. Furthermore, enzymatic and biochemical analysis of the conditional mutant provided strong evidences supporting the notion that AccD5 and/or AccE5 have an additional role in the carboxylation of long chain acyl-CoA prior to mycolic acid condensation. These studies represent a significant step towards a better understanding of the roles of ACCases in mycobacteria and confirm ACCase 5 as an interesting target for the development of new antimycobacterial drugs.
Conflict of interest statement
Figures



Similar articles
-
Functional reconstitution of the Mycobacterium tuberculosis long-chain acyl-CoA carboxylase from multiple acyl-CoA subunits.FEBS J. 2017 Apr;284(7):1110-1125. doi: 10.1111/febs.14046. Epub 2017 Mar 19. FEBS J. 2017. PMID: 28222482 Free PMC article.
-
ACCase 6 is the essential acetyl-CoA carboxylase involved in fatty acid and mycolic acid biosynthesis in mycobacteria.Microbiology (Reading). 2009 Aug;155(Pt 8):2664-2675. doi: 10.1099/mic.0.027714-0. Epub 2009 May 7. Microbiology (Reading). 2009. PMID: 19423629 Free PMC article.
-
The influence of AccD5 on AccD6 carboxyltransferase essentiality in pathogenic and non-pathogenic Mycobacterium.Sci Rep. 2017 Feb 16;7:42692. doi: 10.1038/srep42692. Sci Rep. 2017. PMID: 28205597 Free PMC article.
-
Biochemistry and molecular genetics of cell-wall lipid biosynthesis in mycobacteria.Mol Microbiol. 1997 Apr;24(2):263-70. doi: 10.1046/j.1365-2958.1997.3361705.x. Mol Microbiol. 1997. PMID: 9159514 Review.
-
Regulation and structure of the heteromeric acetyl-CoA carboxylase.Biochim Biophys Acta. 2016 Sep;1861(9 Pt B):1207-1213. doi: 10.1016/j.bbalip.2016.04.004. Epub 2016 Apr 16. Biochim Biophys Acta. 2016. PMID: 27091637 Review.
Cited by
-
Promoter mutagenesis for fine-tuning expression of essential genes in Mycobacterium tuberculosis.Microb Biotechnol. 2018 Jan;11(1):238-247. doi: 10.1111/1751-7915.12875. Epub 2017 Oct 27. Microb Biotechnol. 2018. PMID: 29076636 Free PMC article.
-
Comparative analysis of genomic characteristics and immune response between Mycobacterium tuberculosis strains cultured continuously for 25 years and H37Rv.Pathog Dis. 2024 Feb 7;82:ftae014. doi: 10.1093/femspd/ftae014. Pathog Dis. 2024. PMID: 38845379 Free PMC article.
-
Phylogenetic analysis of vitamin B12-related metabolism in Mycobacterium tuberculosis.Front Mol Biosci. 2015 Mar 4;2:6. doi: 10.3389/fmolb.2015.00006. eCollection 2015. Front Mol Biosci. 2015. PMID: 25988174 Free PMC article. Review.
-
Impaired fatty acid import or catabolism in macrophages restricts intracellular growth of Mycobacterium tuberculosis.Elife. 2025 Mar 13;13:RP102980. doi: 10.7554/eLife.102980. Elife. 2025. PMID: 40080408 Free PMC article.
-
Impaired fatty acid import or catabolism in macrophages restricts intracellular growth of Mycobacterium tuberculosis.bioRxiv [Preprint]. 2025 Jan 11:2024.07.22.604660. doi: 10.1101/2024.07.22.604660. bioRxiv. 2025. Update in: Elife. 2025 Mar 13;13:RP102980. doi: 10.7554/eLife.102980. PMID: 39091727 Free PMC article. Updated. Preprint.
References
-
- Zumla A, Raviglione MC, Hafner R, von Reyn C (2013) Tuberculosis. New Engl J Med 368: 745–755. - PubMed
-
- Cegielski JP (2010) Extensively drug-resistant tuberculosis: “there must be some kind of way out of here”. Clin Infect Dis 50 Suppl 3: S195–200. - PubMed
-
- Harrington M (2010) From HIV to tuberculosis and back again: a tale of activism in 2 pandemics. Clin Infect Dis 50 Suppl 3: S260–266. - PubMed
-
- Daffe M (2008) The global architecture of the mycobacteria cell envelope. In The Mycobacterial Cell Envelope. Daffe M and Reyrat JM (eds). Washington, DC: ASM Press, pp. 3–12.
-
- Brennan PJ, Nikaido H (1995) The envelope of mycobacteria. Annu Rev Biochem 64: 29–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

