Interplay between Candida albicans and the antimicrobial peptide armory
- PMID: 24951441
- PMCID: PMC4135787
- DOI: 10.1128/EC.00093-14
Interplay between Candida albicans and the antimicrobial peptide armory
Abstract
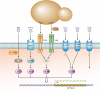
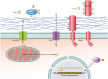
Antimicrobial peptides (AMPs) are key elements of innate immunity, which can directly kill multiple bacterial, viral, and fungal pathogens. The medically important fungus Candida albicans colonizes different host niches as part of the normal human microbiota. Proliferation of C. albicans is regulated through a complex balance of host immune defense mechanisms and fungal responses. Expression of AMPs against pathogenic fungi is differentially regulated and initiated by interactions of a variety of fungal pathogen-associated molecular patterns (PAMPs) with pattern recognition receptors (PRRs) on human cells. Inflammatory signaling and other environmental stimuli are also essential to control fungal proliferation and to prevent parasitism. To persist in the host, C. albicans has developed a three-phase AMP evasion strategy, including secretion of peptide effectors, AMP efflux pumps, and regulation of signaling pathways. These mechanisms prevent C. albicans from the antifungal activity of the major AMP classes, including cathelicidins, histatins, and defensins leading to a basal resistance. This minireview summarizes human AMP attack and C. albicans resistance mechanisms and current developments in the use of AMPs as antifungal agents.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

