Intra-articular enzyme replacement therapy with rhIDUA is safe, well-tolerated, and reduces articular GAG storage in the canine model of mucopolysaccharidosis type I
- PMID: 24951454
- PMCID: PMC4122635
- DOI: 10.1016/j.ymgme.2014.05.015
Intra-articular enzyme replacement therapy with rhIDUA is safe, well-tolerated, and reduces articular GAG storage in the canine model of mucopolysaccharidosis type I
Abstract
Background: Treatment with intravenous enzyme replacement therapy and hematopoietic stem cell transplantation for mucopolysaccharidosis (MPS) type I does not address joint disease, resulting in persistent orthopedic complications and impaired quality of life. A proof-of-concept study was conducted to determine the safety, tolerability, and efficacy of intra-articular recombinant human iduronidase (IA-rhIDUA) enzyme replacement therapy in the canine MPS I model.
Methods: Four MPS I dogs underwent monthly rhIDUA injections (0.58 mg/joint) into the right elbow and knee for 6 months. Contralateral elbows and knees concurrently received normal saline. No intravenous rhIDUA therapy was administered. Monthly blood counts, chemistries, anti-rhIDUA antibody titers, and synovial fluid cell counts were measured. Lysosomal storage of synoviocytes and chondrocytes, synovial macrophages and plasma cells were scored at baseline and 1 month following the final injection.
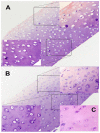
Results: All injections were well-tolerated without adverse reactions. One animal required prednisone for spinal cord compression. There were no clinically significant abnormalities in blood counts or chemistries. Circulating anti-rhIDUA antibody titers gradually increased in all dogs except the prednisone-treated dog; plasma cells, which were absent in all baseline synovial specimens, were predominantly found in synovium of rhIDUA-treated joints at study-end. Lysosomal storage in synoviocytes and chondrocytes following 6 months of IA-rhIDUA demonstrated significant reduction compared to tissues at baseline, and saline-treated tissues at study-end. Mean joint synovial GAG levels in IA-rhIDUA joints were 8.62 ± 5.86 μg/mg dry weight and 21.6 ± 10.4 μg/mg dry weight in control joints (60% reduction). Cartilage heparan sulfate was also reduced in the IA-rhIDUA joints (113 ± 39.5 ng/g wet weight) compared to saline-treated joints (142 ± 56.4 ng/g wet weight). Synovial macrophage infiltration, which was present in all joints at baseline, was abolished in rhIDUA-treated joints only.
Conclusions: Intra-articular rhIDUA is well-tolerated and safe in the canine MPS I animal model. Qualitative and quantitative assessments indicate that IA-rhIDUA successfully reduces tissue and cellular GAG storage in synovium and articular cartilage, including cartilage deep to the articular surface, and eliminates inflammatory macrophages from synovial tissue.
Clinical relevance: The MPS I canine IA-rhIDUA results suggest that clinical studies should be performed to determine if IA-rhIDUA is a viable approach to ameliorating refractory orthopedic disease in human MPS I.
Keywords: Canine; Lysosomal storage disorder; Model; Mucopolysaccharidosis; Orthopedic; Therapy.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I.J Clin Invest. 2008 Aug;118(8):2868-76. doi: 10.1172/JCI34676. J Clin Invest. 2008. PMID: 18654665 Free PMC article.
-
Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I.Mol Genet Metab. 2004 Sep-Oct;83(1-2):163-74. doi: 10.1016/j.ymgme.2004.07.003. Mol Genet Metab. 2004. PMID: 15464431
-
Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I.Biochem Mol Med. 1996 Aug;58(2):156-67. doi: 10.1006/bmme.1996.0044. Biochem Mol Med. 1996. PMID: 8812735
-
Mucopolysaccharidosis type I.Pediatr Endocrinol Rev. 2014 Sep;12 Suppl 1:102-6. Pediatr Endocrinol Rev. 2014. PMID: 25345091 Review.
-
Laronidase treatment of mucopolysaccharidosis I.BioDrugs. 2005;19(1):1-7. doi: 10.2165/00063030-200519010-00001. BioDrugs. 2005. PMID: 15691212 Review.
Cited by
-
Canine Models of Inherited Musculoskeletal and Neurodegenerative Diseases.Front Vet Sci. 2020 Mar 11;7:80. doi: 10.3389/fvets.2020.00080. eCollection 2020. Front Vet Sci. 2020. PMID: 32219101 Free PMC article. Review.
-
Evaluation of tendon and ligament microstructure and mechanical properties in a canine model of mucopolysaccharidosis I.J Orthop Res. 2024 Jul;42(7):1409-1419. doi: 10.1002/jor.25813. Epub 2024 Feb 18. J Orthop Res. 2024. PMID: 38368531 Free PMC article.
-
Diffusion tensor imaging and myelin composition analysis reveal abnormal myelination in corpus callosum of canine mucopolysaccharidosis I.Exp Neurol. 2015 Nov;273:1-10. doi: 10.1016/j.expneurol.2015.07.021. Epub 2015 Jul 26. Exp Neurol. 2015. PMID: 26222335 Free PMC article.
-
The effect of Tlr4 and/or C3 deficiency and of neonatal gene therapy on skeletal disease in mucopolysaccharidosis VII mice.Mol Genet Metab. 2015 Feb;114(2):209-16. doi: 10.1016/j.ymgme.2014.12.305. Epub 2014 Dec 19. Mol Genet Metab. 2015. PMID: 25559179 Free PMC article.
-
Hurdles in treating Hurler disease: potential routes to achieve a "real" cure.Blood Adv. 2020 Jun 23;4(12):2837-2849. doi: 10.1182/bloodadvances.2020001708. Blood Adv. 2020. PMID: 32574368 Free PMC article. Review.
References
-
- Kakkis ED, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, Hasson CW, O'Malley T, Weil MA, Aguirre GA, Brown DE, Haskins ME. Enzyme replacement therapy in feline mucopolysaccharidosis I. Mol. Genet. Metab. 2001;72:199–208. - PubMed
-
- Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, Bleyzac N, Mulier C, Durin A, Kebaili K, Galambrun C, Bertrand Y, Froissart R, Dorche C, Gebuhrer L, Garin C, Berard J, Guibaud P. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. - PubMed
-
- Byers S, Crawley AC, Brumfield LK, Nuttall JD, Hopwood JJ. Enzyme replacement therapy in a feline model of MPS VI: modification of enzyme structure and dose frequency. Pediatr. Res. 2000;47:743–749. - PubMed
-
- Auclair D D, Hein LK, Hopwood JJ, Byers S. Intra-articular enzyme administration for joint disease in feline mucopolysaccharidosis VI: enzyme dose and interval. Pediatr. Res. 2006;59:538–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

