FOLFOX-4 chemotherapy for patients with unresectable or relapsed peritoneal pseudomyxoma
- PMID: 24951608
- PMCID: PMC4122474
- DOI: 10.1634/theoncologist.2014-0106
FOLFOX-4 chemotherapy for patients with unresectable or relapsed peritoneal pseudomyxoma
Abstract
Purpose: The standard treatment of peritoneal pseudomyxoma is based on cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC). The establishment of newer systemic treatments is an unmet clinical need for unresectable or relapsed peritoneal pseudomyxoma. The aim of our study was to assess the activity of chemotherapy with 5-fluorouracil and oxaliplatin (FOLFOX-4 regimen) in terms of response rate in this subset of patients.
Materials and methods: Patients were included in a single-center, observational study and treated with FOLFOX-4 administered every 2 weeks for up to 12 cycles or until progressive disease or unacceptable toxicity.
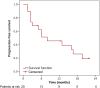
Results: Twenty consecutive patients were reviewed from July 2011 to September 2013. Only partial responses were observed, with an objective response rate of 20%. Median progression-free survival and overall survival were 8 months and 26 months, respectively. Two patients were able to undergo laparotomy with complete cytoreduction and HIPEC in one case. Safety data for FOLFOX-4 were consistent with the literature. By means of a mutant enriched polymerase chain reaction, KRAS mutation was found in 16 of 19 cases (84%), and MGMT promoter methylation was found in 8 (42%, all KRAS mutant).
Conclusion: FOLFOX-4 chemotherapy is tolerable and active in patients with peritoneal pseudomyxoma when disease is deemed unresectable or relapsed after peritonectomy and HIPEC. The identification of predictive biomarkers, such as KRAS for resistance to anti-epidermal growth factor receptor monoclonal antibodies and MGMT for response to temozolomide, is a priority for the development of evidence-based treatment strategies for peritoneal pseudomyxoma.
Keywords: Appendiceal cancer; Chemotherapy; FOLFOX-4; Peritoneal pseudomyxoma.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Comment in
-
Challenges of efficacy assessments in pseudomyxoma peritonea.Oncologist. 2015 Mar;20(3):e3-4. doi: 10.1634/theoncologist.2014-0415. Oncologist. 2015. PMID: 25745054 Free PMC article.
-
In reply.Oncologist. 2015 Mar;20(3):e5. doi: 10.1634/theoncologist.2014-423. Oncologist. 2015. PMID: 25745055 Free PMC article.
References
-
- Smeenk RM, van Velthuysen ML, Verwaal VJ, et al. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur J Surg Oncol. 2008;34:196–201. - PubMed
-
- et al. In: WHO Classification of Tumours of the Digestive System. 4th ed. Bosman FT, Carneiro F, Hruban RH, editors; Lyon, France: International Agency for Research on Cancer; 2010.
-
- Bradley RF, Stewart JH, IV, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: A clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–559. - PubMed
-
- Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous