Quality of life in the trastuzumab for gastric cancer trial
- PMID: 24951609
- PMCID: PMC4077451
- DOI: 10.1634/theoncologist.2014-0058
Quality of life in the trastuzumab for gastric cancer trial
Abstract
Background: The Trastuzumab for Gastric Cancer phase III trial demonstrated that combining trastuzumab with chemotherapy significantly improved overall survival compared with chemotherapy alone in HER2-positive advanced gastric or gastroesophageal junction cancer. We report health-related quality of life (HRQoL) and quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) results from this trial.
Patients and methods: Patients were randomized to receive six cycles of chemotherapy given every 3 weeks (capecitabine or fluorouracil, plus cisplatin) either alone or combined with administration of trastuzumab every 3 weeks until disease progression. At each clinical visit, HRQoL was assessed using two European Organization for Research and Treatment of Cancer quality of life questionnaires, QLQ-C30 and QLQ-STO22. Q-TWiST methodology was applied retrospectively using the clinical data and utility coefficients.
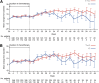
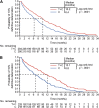
Results: Trastuzumab plus chemotherapy prolonged time to 10% definitive deterioration in all QLQ-C30 and QLQ-STO22 scores, including QLQ-C30 global health status versus chemotherapy alone, from 6.4 months to 10.2 months. In addition, trastuzumab plus chemotherapy extended Q-TWiST by 2.42 months compared with chemotherapy alone.
Conclusion: Compared with chemotherapy alone, trastuzumab plus chemotherapy prolongs time to deterioration of HRQoL and increases quality-adjusted survival in patients with HER2-positive gastric or gastroesophageal junction cancer.
摘要
背景。使用曲妥珠单抗治疗胃癌的 III 期研究表明,与单纯化疗相比,曲妥珠单抗与化疗药物的联合使用可显著提高 HER2 阳性晚期胃癌或食管胃结合部癌患者的总生存期。本文报告了这项研究的健康相关生存质量 (HRQoL) 及生存质量校正后的无疾病症状无毒性生存期 (Q-TWiST) 结果。
患者与方法。患者被随机分为两组,分别接受单纯化疗(卡培他滨或氟尿嘧啶,联合顺铂,每 3 周一个周期,共 6 个周期)或化疗联合曲妥珠单抗(每 3 周一次)治疗,直至出现疾病进展。在每次临床就诊时,我们采用欧洲肿瘤研治组织的两份生存质量调查问卷 QLQ-C30 和 QLQ-STO22 对受试者进行 HRQoL 评估。Q-TWiST 的评估则采用回顾性方式依据临床数据和效用系数来进行。
结果。与单纯化疗相比,曲妥珠单抗与化疗药物的联合使用将所有 QLQ-C30 和 QLQ-STO22 评估指标的“至 10% 确定性下降时间”从 6.4 个月推迟到了 10.2 个月,其中包括 QLQ-C30 总体健康状态这一指标。此外,与单纯化疗相比,曲妥珠单抗与化疗的联合使用也将 Q-TWiST 延长了 2.42 个月。
结论。与单纯化疗相比,曲妥珠单抗与化疗的联合使用推迟了 HRQoL 的至下降时间,提高了 HER2 阳性胃癌或食管胃结合部癌患者的生存质量校正后生存期。
The Oncologist 2014;19:712–719
Trial registration: ClinicalTrials.gov NCT01041404.
Keywords: Chemotherapy; Gastric cancer; HER2; Quality of life; Trastuzumab.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures


References
-
- GLOBOCAN stomach cancer fact sheet 2012. Available at http://globocan.iarc.fr/old/FactSheets/cancers/stomach-new.asp Accessed May 23, 2014.
-
- Kang H, Kauh JS. Chemotherapy in the treatment of metastatic gastric cancer: Is there a global standard? Curr Treat Options Oncol. 2011;12:96–106. - PubMed
-
- Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: A practical approach. Mod Pathol. 2012;25:637–650. - PubMed
-
- Albarello L, Pecciarini L, Doglioni C. HER2 testing in gastric cancer. Adv Anat Pathol. 2011;18:53–59. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

