Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements
- PMID: 24951618
- PMCID: PMC4146372
- DOI: 10.1113/jphysiol.2014.274456
Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements
Abstract
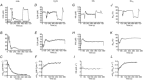
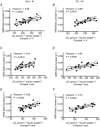
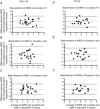
The present study aimed to compare in vivo measurements of skeletal muscle mitochondrial respiratory capacity made using near-infrared spectroscopy (NIRS) with the current gold standard, namely in situ measurements of high-resolution respirometry performed in permeabilized muscle fibres prepared from muscle biopsies. Mitochondrial respiratory capacity was determined in 21 healthy adults in vivo using NIRS to measure the recovery kinetics of muscle oxygen consumption following a ∼15 s isometric contraction of the vastus lateralis muscle. Maximal ADP-stimulated (State 3) respiration was measured in permeabilized muscle fibres using high-resolution respirometry with sequential titrations of saturating concentrations of metabolic substrates. Overall, the in vivo and in situ measurements were strongly correlated (Pearson's r = 0.61-0.74, all P < 0.01). Bland-Altman plots also showed good agreement with no indication of bias. The results indicate that in vivo NIRS corresponds well with the current gold standard, in situ high-resolution respirometry, for assessing mitochondrial respiratory capacity.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures
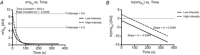
References
-
- Belardinelli R, Barstow TJ, Nguyen P, Wasserman K. Skeletal muscle oxygenation and oxygen uptake kinetics following constant work rate exercise in chronic congestive heart failure. Am J Cardiol. 1997;80:1319–1324. - PubMed
-
- Binzoni T, Ferretti G, Schenker K, Cerretelli P. Phosphocreatine hydrolysis by 31P-NMR at the onset of constant-load exercise in humans. J Appl Physiol (1985) 1992;73:1644–1649. - PubMed
-
- Boone J, Koppo K, Barstow TJ, Bouckaert J. Pattern of deoxy[Hb+Mb] during ramp cycle exercise: influence of aerobic fitness status. Eur J Appl Physiol. 2009;105:851–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

