Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes
- PMID: 24951641
- PMCID: PMC4107739
- DOI: 10.1093/brain/awu142
Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes
Erratum in
- Brain. 2014 Dec;137(Pt 12):e315. Press, Raomand [corrected to Press, Rayomand]
-
Editorial.Brain. 2014 Jan;137(Pt 1):1. doi: 10.1093/brain/awt349. Epub 2013 Dec 25. Brain. 2014. PMID: 24371216 Free PMC article. No abstract available.
Abstract
The clinical associations of glycine receptor antibodies have not yet been described fully. We identified prospectively 52 antibody-positive patients and collated their clinical features, investigations and immunotherapy responses. Serum glycine receptor antibody endpoint titres ranged from 1:20 to 1:60 000. In 11 paired samples, serum levels were higher than (n = 10) or equal to (n = 1) cerebrospinal fluid levels; there was intrathecal synthesis of glycine receptor antibodies in each of the six pairs available for detailed study. Four patients also had high glutamic acid decarboxylase antibodies (>1000 U/ml), and one had high voltage-gated potassium channel-complex antibody (2442 pM). Seven patients with very low titres (<1:50) and unknown or alternative diagnoses were excluded from further study. Three of the remaining 45 patients had newly-identified thymomas and one had a lymphoma. Thirty-three patients were classified as progressive encephalomyelitis with rigidity and myoclonus, and two as stiff person syndrome; five had a limbic encephalitis or epileptic encephalopathy, two had brainstem features mainly, two had demyelinating optic neuropathies and one had an unclear diagnosis. Four patients (9%) died during the acute disease, but most showed marked improvement with immunotherapies. At most recent follow-up, (2-7 years, median 3 years, since first antibody detection), the median modified Rankin scale scores (excluding the four deaths) decreased from 5 at maximal severity to 1 (P < 0.0001), but relapses have occurred in five patients and a proportion are on reducing steroids or other maintenance immunotherapies as well as symptomatic treatments. The glycine receptor antibodies activated complement on glycine receptor-transfected human embryonic kidney cells at room temperature, and caused internalization and lysosomal degradation of the glycine receptors at 37°C. Immunoglobulin G antibodies bound to rodent spinal cord and brainstem co-localizing with monoclonal antibodies to glycine receptor-α1. Ten glycine receptor antibody positive samples were also identified in a retrospective cohort of 56 patients with stiff person syndrome and related syndromes. Glycine receptor antibodies are strongly associated with spinal and brainstem disorders, and the majority of patients have progressive encephalomyelitis with rigidity and myoclonus. The antibodies demonstrate in vitro evidence of pathogenicity and the patients respond well to immunotherapies, contrasting with earlier studies of this syndrome, which indicated a poor prognosis. The presence of glycine receptor antibodies should help to identify a disease that responds to immunotherapies, but these treatments may need to be sustained, relapses can occur and maintenance immunosuppression may be required.
Keywords: autoantibody; autoimmune encephalitis; glycine receptor; progressive encephalomyelitis with rigidity and myoclonus; stiff person syndrome.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

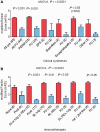
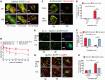
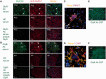
Comment in
-
Glycine receptor antibodies in PERM: a new channelopathy.Brain. 2014 Aug;137(Pt 8):2115-6. doi: 10.1093/brain/awu153. Brain. 2014. PMID: 25057131 Free PMC article.
References
-
- Alexopoulos H, Dalakas MC. A critical update on the immunopathogenesis of Stiff Person Syndrome. Eur J Clin Invest. 2010;40:1018–25. - PubMed
-
- Alexopoulos H, Akrivou S, Dalakas MC. Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders. Neurology. 2013;81:1962–4. - PubMed
-
- Angus-Leppan H, Rudge P, Mead S, Collinge J, Vincent A. Autoantibodies in sporadic Creutzfeldt-Jakob disease. JAMA Neurol. 2013;70:919–22. - PubMed
-
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–24. - PubMed
-
- Bourke D, Roxburgh R, Vincent A, Cleland J, Jeffery O, Dugan N, et al. Hypoventilation in glycine-receptor antibody related progressive encephalomyelitis, rigidity and myoclonus. J Clin Neurosci. 2014;21:876–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

