In situ determination of alveolar septal strain, stress and effective Young's modulus: an experimental/computational approach
- PMID: 24951778
- PMCID: PMC4137161
- DOI: 10.1152/ajplung.00106.2014
In situ determination of alveolar septal strain, stress and effective Young's modulus: an experimental/computational approach
Abstract
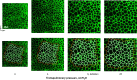
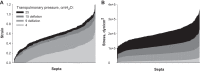
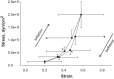
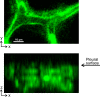
Alveolar septa, which have often been modeled as linear elements, may distend due to inflation-induced reduction in slack or increase in tissue stretch. The distended septum supports tissue elastic and interfacial forces. An effective Young's modulus, describing the inflation-induced relative displacement of septal end points, has not been determined in situ for lack of a means of determining the forces supported by septa in situ. Here we determine such forces indirectly according to Mead, Takishima, and Leith's classic lung mechanics analysis (J Appl Physiol 28: 596-608, 1970), which demonstrates that septal connections transmit the transpulmonary pressure, PTP, from the pleural surface to interior regions. We combine experimental septal strain determination and computational stress determination, according to Mead et al., to calculate effective Young's modulus. In the isolated, perfused rat lung, we label the perfusate with fluorescence to visualize the alveolar septa. At eight PTP values around a ventilation loop between 4 and 25 cmH2O, and upon total deflation, we image the same region by confocal microscopy. Within an analysis region, we measure septal lengths. Normalizing by unstressed lengths at total deflation, we calculate septal strains for all PTP > 0 cmH2O. For the static imaging conditions, we computationally model application of PTP to the boundary of the analysis region and solve for septal stresses by least squares fit of an overdetermined system. From group septal strain and stress values, we find effective septal Young's modulus to range from 1.2 × 10(5) dyn/cm(2) at low P(TP) to 1.4 × 10(6) dyn/cm(2) at high P(TP).
Keywords: alveolar septum; intact lung; modulus; strain; stress.
Copyright © 2014 the American Physiological Society.
Figures





References
-
- Bachofen H, Schürch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol 62: 1878–1887, 1987 - PubMed
-
- Cavalcante FSA, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, Andrade JS, Jr, Majumdar A, Ingenito EP, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol 98: 672–679, 2005 - PubMed
-
- Fukaya H, Martin CJ, Young AC, Katsura S. Mechanical properties of alveolar walls. J Appl Physiol 25: 689–695, 1968 - PubMed
-
- Fung YC. Biomechanics: Mechanical Properties of Living Tissues (2nd ed.) New York: Springer-Verlag, 1993
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

