A novel intramuscular bivalent norovirus virus-like particle vaccine candidate--reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults
- PMID: 24951828
- PMCID: PMC8483568
- DOI: 10.1093/infdis/jiu337
A novel intramuscular bivalent norovirus virus-like particle vaccine candidate--reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults
Abstract
Background: Noroviruses are the most important viral causes of gastroenteritis-related morbidity and mortality. A randomized, double-blind, placebo-controlled study evaluated an adjuvanted bivalent intramuscular norovirus virus-like particle (VLP) vaccine.
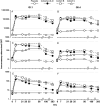
Methods: Forty-eight adults aged 18-49 years received either 2 doses containing genotype GI.1 VLP and a consensus GII.4 VLP or 2 doses of placebo. Doses (5 µg, 15 µg, 50 µg, or 150 µg of each VLP) were administered 4 weeks apart in the first stage. Subsequently, 54 adults, aged 18-49 (n=16), 50-64 (n=19), and 65-85 (n=19) years, received 2 doses of vaccine containing 50 µg of each VLP. Total and class-specific antibody responses, as well as histoblood group antigen (HBGA) blocking antibody responses, were measured before and after each dose.
Results: Local reactions were mainly injection site pain/tenderness, with no reported fever or vaccine-related serious adverse events. One dose of vaccine containing 50 µg of each VLP increased GI.1 geometric mean titers (GMTs) by 118-fold, 83-fold, and 24-fold and increased GII.4 GMTs by 49-fold, 25-fold, and 9-fold in subjects aged 18-49, 50-64, and 65-83 years, respectively. Serum antibody responses peaked at day 7 after the first dose, with no evidence of boosting following a second dose. Most subjects achieved HBGA-blocking antibody titers of ≥200.
Conclusions: The vaccine was well tolerated and immunogenic. Rapid immune response to a single dose may be particularly useful in military personnel and travelers and in the control of outbreaks. Clinical Trials Registration. NCT01168401.
Keywords: gastroenteritis; norovirus; vaccine; virus-like particle.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis. 2011;52:466–74. - PubMed
-
- Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and Norovirus among gastroenteritis-associated deaths in the United States 199–2007. Clin Infect Dis. 2012;55:213–23. - PubMed
-
- Tacket CO, Sztein MB, Losonsky G, Wasserman S, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

