Antimicrobial peptides from scorpion venoms
- PMID: 24951876
- PMCID: PMC7111748
- DOI: 10.1016/j.toxicon.2014.06.006
Antimicrobial peptides from scorpion venoms
Abstract
The need for new antimicrobial agents is becoming one of the most urgent requirements in modern medicine. The venoms of many different species are rich sources of biologically active components and various therapeutic agents have been characterized including antimicrobial peptides (AMPs). Due to their potent activity, low resistance rates and unique mode of action, AMPs have recently received much attention. This review focuses on AMPs from the venoms of scorpions and examines all classes of AMPs found to date. It gives details of their biological activities with reference to peptide structure. The review examines the mechanism of action of AMPs and with this information, suggests possible mechanisms of action of less well characterised peptides. Finally, the review examines current and future trends of scorpion AMP research, by discussing recent successes obtained through proteomic and transcriptomic approaches.
Keywords: Infection; Pore forming peptides; Scorpion venom; Therapeutics; Venomics.
Copyright © 2014. Published by Elsevier Ltd.
Figures
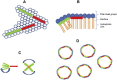
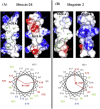
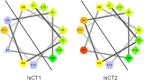
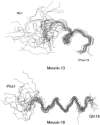


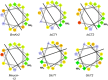
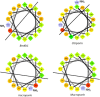

References
-
- Abdel-Rahman M.A., Quintero-Hernandez V., Possani L.D. Venom proteomic and venomous glands transcriptomic analysis of the Egyptian scorpion Scorpio maurus palmatus (Arachnida: Scorpionidae) Toxicon. 2013;74:193–207. - PubMed
-
- Abdel-Rahman M.A., Harrison P.L., Strong P.N. Snapshots of scorpion venomics. J. Arid Environ. 2014 (in press)
-
- Abdel-Rahman M.A., Omran M., Abdel-Nabi M., Ueda H., McVean A. Intraspecific variation in the Egyptian Scorpion Scorpio maurus palmatus venom collected from different biotopes. Toxicon. 2009;53:349–359. - PubMed
-
- Almaaytah A., Zhou M., Wang L., Chen T., Walker B., Shaw C. Antimicrobial/cytolytic peptides from the venom of the North African scorpion, Androctonus amoreuxi: biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides. 2012;35:291–299. - PubMed
Further reading
-
- Borges A., Garcia C.C., Lugo E., Alfonzo M.J., Jowers M.J., Op den Camp H.J.M. Diversity of long-chain toxins in Tityus zulianus and Tityus discrepans venoms (Scorpiones, Buthidae): molecular, immunological, and mass spectral analyses. Comp. Biochem Physiol. 2006;142C:240–252. - PubMed
-
- Morgenstern D., Rohde B.H., King G.F., Tal T., Sher D., Zlotkin E. The tale of a resting gland: transcriptome of a replete venom gland from the scorpion Hottentotta judaicus. Toxicon. 2011;57:695–703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

