Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: relationship with survival-a substudy of the MIMOSA trial
- PMID: 24952307
- PMCID: PMC11029557
- DOI: 10.1007/s00262-014-1569-0
Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: relationship with survival-a substudy of the MIMOSA trial
Abstract
Purpose: To determine whether abagovomab induces protective immune responses in ovarian cancer patients in first clinical remission. The present analysis is a substudy of monoclonal antibody immunotherapy for malignancies of the ovary by subcutaneous abagovomab trial (NCT00418574).
Methods: The study included 129 patients, 91 in the abagovomab arm and 38 in the placebo arm. Circulating CA125-specific cytotoxic T lymphocytes (CTL) were measured by a flow cytometry-based interferon-γ producing assay. Human antimouse antibody and anti-anti-idiotypic (Ab3) were assessed by ELISA. Patients were evaluated before starting the treatment and at different time points during induction and maintenance phases.
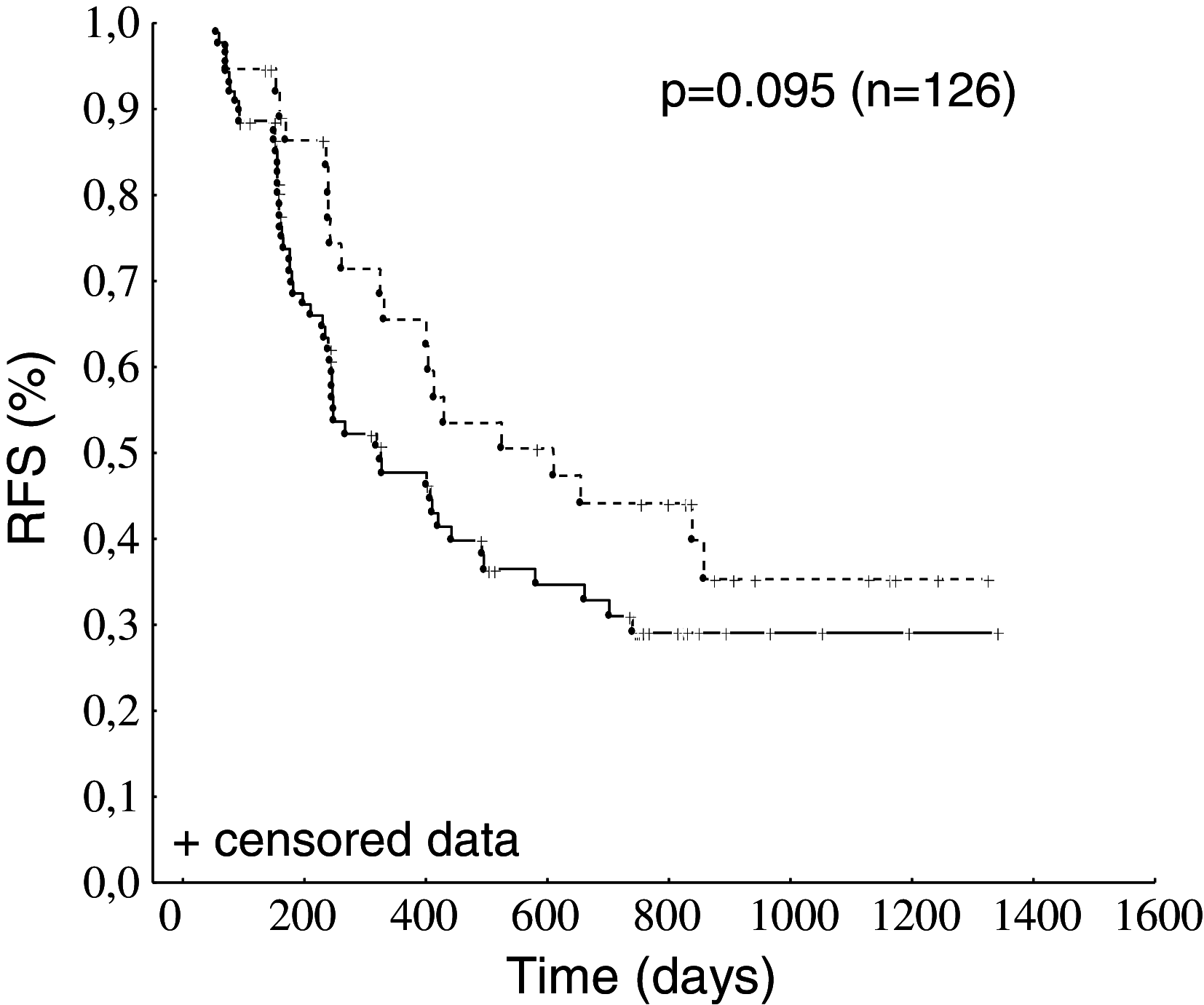
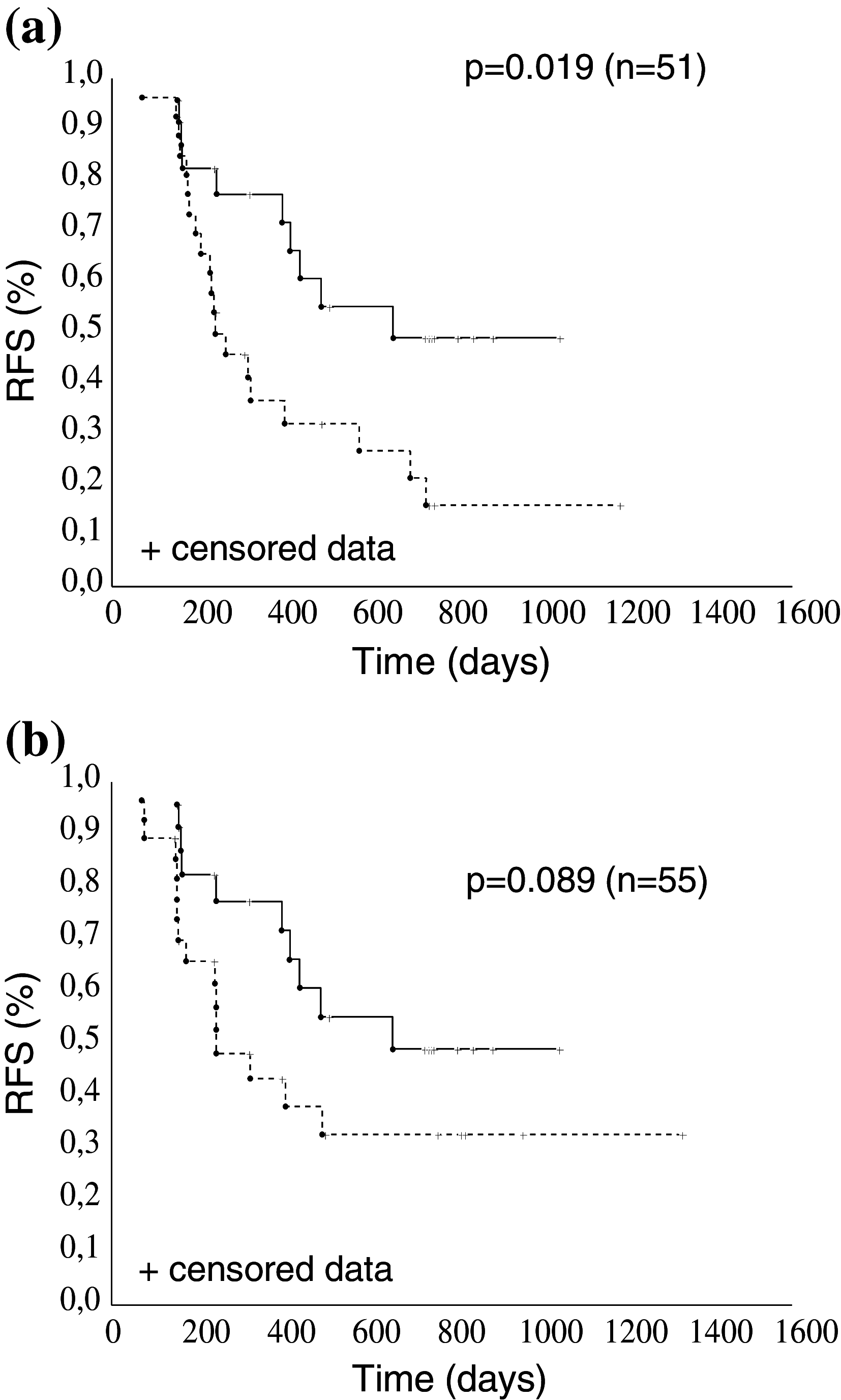
Results: A similar percentage of patients in both the placebo and abagovomab arms had CA125-specific CTL (26.3 and 31.8 %, respectively; p = 0.673 by Fisher's exact test). Patients with CA125-specific CTL in both arms tended to have an increased relapse-free survival (RFS, log-rank test p = 0.095) compared to patients without. Patients (n = 27) in the abagovomab arm without CA125-specific CTL but that developed Ab3 above the cutoff (defined as median Ab3 level at week 22) had a prolonged RFS compared to patients (n = 24) that did not develop Ab3 above the cutoff (log-rank test p = 0.019).
Conclusion: Abagovomab does not induce CA125-specific CTL. However, patients with CA125-specific CTL perform better than patients without, irrespective of abagovomab treatment. Abagovomab-induced Ab3 associate with prolonged RFS in patients without CA125-specific CTL. Further studies are needed to confirm these data and to assess the potential utility of these immunological findings as a tool for patient selection in clinical trial.
Conflict of interest statement
Part of reagents (e.g., mAbs, CA125, staining buffers, etc.) and disposables (plasticwares) have been provided by Menarini Ricerche, Pomezia, Italy. The authors declare they have no financial or other interest that is relevant to the subject matter under consideration in this article with Menarini Ricerche.
Figures


Similar articles
-
A robust immune system conditions the response to abagovomab (anti-idiotypic monoclonal antibody mimicking the CA125 protein) vaccination in ovarian cancer patients.Immunol Lett. 2017 Nov;191:35-39. doi: 10.1016/j.imlet.2017.09.006. Epub 2017 Sep 14. Immunol Lett. 2017. PMID: 28919454 Clinical Trial.
-
Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a phase III trial of the AGO OVAR, COGI, GINECO, and GEICO--the MIMOSA study.J Clin Oncol. 2013 Apr 20;31(12):1554-61. doi: 10.1200/JCO.2012.46.4057. Epub 2013 Mar 11. J Clin Oncol. 2013. PMID: 23478059 Free PMC article. Clinical Trial.
-
The anti-idiotypic antibody abagovomab in patients with recurrent ovarian cancer. A phase I trial of the AGO-OVAR.Ann Oncol. 2006 Oct;17(10):1568-77. doi: 10.1093/annonc/mdl357. Ann Oncol. 2006. PMID: 17005631 Clinical Trial.
-
Abagovomab: an anti-idiotypic CA-125 targeted immunotherapeutic agent for ovarian cancer.Immunotherapy. 2011 Feb;3(2):153-62. doi: 10.2217/imt.10.100. Immunotherapy. 2011. PMID: 21322756 Free PMC article. Review.
-
The antibody-based CA125-targeted maintenance therapy for the epithelial ovarian cancer: a meta-analysis.Eur J Gynaecol Oncol. 2016 Aug;37(4):455-460. Eur J Gynaecol Oncol. 2016. PMID: 29894066 Review.
Cited by
-
Feasibility Study of a Network Meta-Analysis and Unanchored Population-Adjusted Indirect Treatment Comparison of Niraparib, Olaparib, and Bevacizumab as Maintenance Therapies in Patients with Newly Diagnosed Advanced Ovarian Cancer.Cancers (Basel). 2022 Mar 2;14(5):1285. doi: 10.3390/cancers14051285. Cancers (Basel). 2022. PMID: 35267593 Free PMC article. Review.
-
Multifaceted glycoadjuvant@AuNPs inhibits tumor metastasis through promoting T cell activation and remodeling tumor microenvironment.J Nanobiotechnology. 2021 Nov 18;19(1):376. doi: 10.1186/s12951-021-01129-3. J Nanobiotechnology. 2021. PMID: 34794428 Free PMC article.
-
Neoantigens retention in patient derived xenograft models mediates autologous T cells activation in ovarian cancer.Oncoimmunology. 2019 Mar 30;8(6):e1586042. doi: 10.1080/2162402X.2019.1586042. eCollection 2019. Oncoimmunology. 2019. PMID: 31069153 Free PMC article.
-
Immunotherapy For Ovarian Cancer: Recent Advances And Combination Therapeutic Approaches.Onco Targets Ther. 2020 Jun 26;13:6109-6129. doi: 10.2147/OTT.S205950. eCollection 2020. Onco Targets Ther. 2020. PMID: 32617007 Free PMC article. Review.
-
Virus-like particle vaccine displaying an external, membrane adjacent MUC16 epitope elicits ovarian cancer-reactive antibodies.J Ovarian Res. 2024 Jan 16;17(1):19. doi: 10.1186/s13048-023-01325-9. J Ovarian Res. 2024. PMID: 38225646 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

